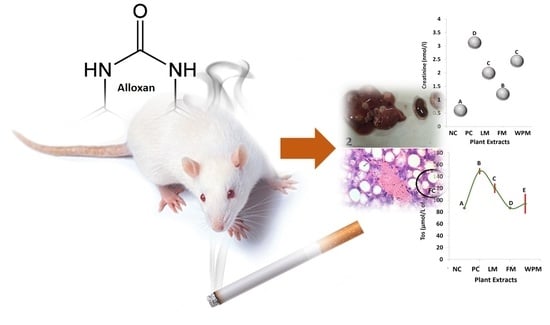
Anti-Diabetic and Cytotoxic Evaluation of Phlomis stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant
2.2. Washing and Separation of Plant Parts
2.3. Washing and Separation of Plant Parts
2.4. LC-ESI-MS Analysis
2.5. Determination of Cytotoxicity
2.6. Cigarette Smoke Inhalation and Alloxan-Induced Diabetes
2.7. Animals Grouping and Dose Administration
2.8. Blood and Organs Collection
2.9. Biochemistry Study
2.10. Statistical Analysis
3. Results
3.1. Extraction Yield
3.2. LC-ESI-MS Chromatogram and Fragmentation Pattern
3.3. Cytotoxicity Assay
3.4. Alteration in Hepatic Dysfunction Restoration
3.5. Improvement in Alterations in Hepatocellular Architecture
3.6. P. stewartii Hf. Alleviates Renal Damage
3.7. Antioxidant Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahzad, A.; Hussain, S.; Anwar, N.; Karim, A.; Aeman, U.; Iqbal, M.J. An overview of free radicals & antioxidants and its deletenous actions. Front. Chem. Sci. 2021, 2, 147–164. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 2170372. [Google Scholar] [CrossRef] [Green Version]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamat, S.S.; Kamarolzaman, M.F.F.; Yahya, F.; Mahmood, N.D.; Shahril, M.S.; Jakius, K.F.; Mohtarrudin, N.; Ching, S.M.; Susanti, D.; Taher, M.; et al. Methanol extract of melastoma malabathricum leaves exerted antioxidant and liver protective activity in rats. BMC Complement. Altern. Med. 2013, 13, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Quan, D.V.; Sung, J.H.; Lee, M.-Y.; Ha, H. Cigarette smoke inhalation aggravates diabetic kidney injury in rats. R. Soc. Chem. 2019, 8, 964–971. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Yahya, F.; Mamat, S.S.; Mahmood, N.D.; Mohtarrudin, N.; Taher, M.; Hamid, S.S.A.; Teh, L.K.; Salleh, M.Z. Hepato-protective action of various partitions of methanol extract of bauhinia purpurea leaves against paracetamol-induced liver toxicity: Involvement of the antioxidant mechanisms. BMC Complement. Altern. Med. 2016, 16, 175. [Google Scholar] [CrossRef] [Green Version]
- Cheemerla, S.; Balakrishnan, M. Global epidemiology of chronic liver disease. Clin. Liver Dis. 2021, 17, 365. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From cirrhosis to hepatocellular carcinoma: New molecular insights on inflammation and cellular senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef]
- Patel, D.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Macdonald Ighodaro, O.; Mohammed Adeosun, A.; Adeboye Akinloye, O. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R. Thioredoxin and thioredoxin target proteins: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Gao, J.; Liang, Z.; Yang, D. Pdi-regulated disulfide bond formation in protein folding and biomolecular assembly. Molecules 2020, 26, 171. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Am. Diabetes Assoc. 2014, 37, S81–S90. [Google Scholar]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diabetes Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Hu, K.; Ren, J.; Martorell, M.; Calina, D.; Cho, W.C.; Sharifi-Rad, J.; Sharifi-Rad, M.; Zucca, P.; Varoni, E. Lifestyle, oxidativestress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar]
- World Health Organization. Who Report on the Global Tobacco Epidemic, 2021: Addressing New And emerging Products; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Afzal, S.; Ahmad, H.I.; Jabbar, A.; Tolba, M.M.; AbouZid, S.; Irm, N.; Zulfiqar, F.; Iqbal, M.Z.; Ahmad, S.; Aslam, Z. Use of medicinal plants for respiratory diseases in bahawalpur, pakistan. BioMed Res. Int. 2021, 2021, 5578914. [Google Scholar] [CrossRef]
- Assiri, A.M. Traditional medicinal plants used for respiratory disorders in pakistan: A review of the ethno-medicinal and pharmacological evidence. Chin. Med. 2018, 13, 48. [Google Scholar]
- Wu, Q.; Jiang, X.; Lu, Q.; Li, J.; Chen, J. Changes in soil organic carbon and aggregate stability following a chronosequence of Liriodendron chinense plantations. J. For. Res. 2021, 32, 355–362. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Available online: http://specimens.kew.org/herbarium/K000894367 (accessed on 29 October 2022).
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Management Medicinal plants of the family lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, B.; Riaz, N.; Saleem, M.; Naveed, M.A.; Ashraf, M.; Alam, U.; Rafiq, H.M.; Tareen, R.B.; Jabbar, A. Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity. Phytochemistry 2013, 96, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Zadeh, M.S.; Mohabatkar, H. Evaluation of antiherpetic activity of crude extract and fractions of avicenna marina, in vitro. Antivir. Res. 2013, 97, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clement, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Off. J. Am. Assoc. Study Liver Dis. 2012, 56, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Juretić, D.; Motejlkova, A.; Kunovic, B.; Rekic, B.; Flegar-Mestric, Z.; Vujic, L.; MESIC, R.; Lukac-Bajalo, J.; Simeon-Rudolf, V. Paraoxonase/arylesterase in serum of patients with type ii diabetes mellitus. Acta Pharm. 2006, 56, 59–68. [Google Scholar]
- Firuzi, O.; Javidnia, K.; Gholami, M.; Soltani, M.; Miri, R. Antioxidant activity and total phenolic content of 24 lamiaceae species growing in iran. Nat. Prod. Commun. 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Geng, D.; Innes, J.; Wu, W.; Wang, G. Impacts of COVID-19 pandemic on urban park visitation: A global analysis. J. Offorestry Res. 2021, 32, 553–567. [Google Scholar] [CrossRef]
- Sarkhail, P.; Sahranavard, S.; Nikan, M.; Gafari, S.; Eslami-Tehrani, B. Evaluation of the cytotoxic activity of extracts from six species of Phlomis Genus. J. Appl. Pharm. Sci. 2017, 7, 180–184. [Google Scholar]
- Sarikurkcu, C.; Uren, M.C.; Tepe, B.; Cengiz, M.; Kocak, M.S. Phlomis armeniaca: Phenolic compounds, enzyme inhibitory and antioxidant activities. Ind. Crops Prod. 2015, 78, 95–101. [Google Scholar] [CrossRef]
- Samar Ali, A.F.; Ullah, H.; Inayat Agha, A.K.N.J.; Tareen, R.B. 30. Evaluation of antioxidant, antimicrobial activity and GC-MS analysis of Phlomis stewartii. Pure Appl. Biol. (PAB) 2019, 8, 2420–2433. [Google Scholar]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2020, 22, 361. [Google Scholar] [CrossRef]
- Zych, M.; Kaczmarczyk-Sedlak, I.; Wojnar, W.; Folwarczna, J. The Effects of Sinapic Acid on the Development of Metabolic Disorders Induced by Estrogen Deficiency in Rats. Oxidative Med. Cell. Longev. 2018, 2018, 9274246. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cellular physiology and biochemistry: International journal of experimental cellular physiology. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxidative Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [Green Version]
- Yun, U.J.; Yang, D.K. Sinapic Acid Inhibits Cardiac Hypertrophy via Activation of Mitochondrial Sirt3/SOD2 Signaling in Neonatal Rat Cardiomyocytes. Antioxidants 2020, 9, 1163. [Google Scholar] [CrossRef]
- Xianchu, L.; Ming, L.; Changhao, C.; Beiwang, D.; Jingtao, X. Sinapic acid attenuates muscle atrophy in streptozotocin-induced diabetic mice. Iran. J. Basic Med. Sci. 2021, 24, 1695–1701. [Google Scholar] [CrossRef]
- Göger, G.; Türkyolu, Ü.; Gürşen, E.N.; Yur, S.; Karaduman, A.B.; Göger, F.; Tekin, M.; Özek, G. Phytochemical characterisation of Phlomis linearis Boiss. & Bal and screening for anticholinesterase, antiamylase, antimicrobial, and cytotoxic properties. Turk. J. Chem. 2021, 45, 387–399. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Khalyfa, A.A.; Punatar, S.; Yarbrough, A. Hepatocellular carcinoma: Understanding the inflammatory implications of the microbiome. Int. J. Mol. Sci. 2022, 23, 8164. [Google Scholar] [CrossRef]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Abu Dayyeh, B.K.; Hurt, R.T. Evolution of nafld and its management. Nutr. Clin. Pract. 2020, 35, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Nsiah, K.; Shang, V.O.; Boateng, K.A.; Mensah, F.; Research, B.M. Prevalence of metabolic syndrome in type 2 diabetes mellitus patients. Int. J. Appl. Basic Med. Res. 2015, 5, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taye, G.M.; Bule, M.; Gadisa, D.A.; Teka, F.; Abula, T. In vivo antidiabetic activity evaluation of aqueous and 80% methanolic extracts of leaves of thymus schimperi (lamiaceae) in alloxan-induced diabetic mice. Targets Ther. 2020, 13, 3205. [Google Scholar]
- Sarkhail, P.; Rahmanipour, S.; Fadyevatan, S.; Mohammadirad, A.; Dehghan, G.; Amin, G.; Shafiee, A.; Abdollahi, M. Antidiabetic effect of P. anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacol. Res. 2007, 56, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Määttä, A.-M.; Salminen, A.; Pietiäinen, M.; Leskelä, J.; Palviainen, T.; Sattler, W.; Sinisalo, J.; Salomaa, V.; Kaprio, J.; Pussinen, P. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. 2021, 27, 3–14. [Google Scholar] [CrossRef]
- Lucchesi, A.N.; Cassettari, L.L.; Spadella, C.T. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. J. Diabetes Res. 2015, 2015, 494578. [Google Scholar] [CrossRef] [Green Version]
- Atawodi, S.E.-O.; Yakubu, O.E.; Liman, M.L.; Iliemene, D.U. Effect of methanolic extract of tetrapleura tetraptera(schum and thonn) taub leaves on hyperglycemia and indices of diabetic complications in alloxan–induced diabetic rats. Asian Pac. J. Trop. Biomed. 2014, 4, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhou, F.; Shen, S.; Zhang, T. Fighting liver fibrosis with naturally occurring antioxidants. Planta Med. 2018, 84, 1318–1333. [Google Scholar] [CrossRef]
- Kong, M.; Xie, K.; Lv, M.; Li, J.; Yao, J.; Yan, K.; Wu, X.; Xu, Y.; Ye, D. Pharmacotherapy Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother. 2021, 133, 110975. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. Metabolic abnormalities in diabetes and kidney disease: Role of uremic toxins. Microvasular Complicat.—Nephrop. 2018, 18, 97. [Google Scholar] [CrossRef]
- Sarkhail, P.; Abdollahi, M.; Fadayevatan, S.; Shafiee, A.; Mohammadirad, A.; Dehghan, G.; Esmaily, H.; Amin, G. Effect of P. persica on glucose levels and hepatic enzymatic antioxidants in streptozotocin-induced diabetic rats. Pharmacogn. Mag. 2010, 6, 219. [Google Scholar] [CrossRef] [Green Version]
- Đurašević, S.; Jasnić, N.; Prokić, M.; Grigorov, I.; Martinović, V.; Đorđević, J.; Pavlović, S. The protective role of virgin coconut oil on the alloxan-induced oxidative stress in the liver, kidneys and heart of diabetic rats. Food Funct. 2019, 10, 2114–2124. [Google Scholar] [CrossRef]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic toxins and their relation with oxidative stress induced in patients with ckd. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C. Liver, oxidative stress and metabolic syndromes. Nutrients 2021, 13, 301. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M. Red-leafed species for urban “greening” in the age of global climate change. J. For. Res. 2021, 32, 151–159. [Google Scholar] [CrossRef]
- Algieri, F.; Zorrilla, P.; Rodriguez-Nogales, A.; Garrido-Mesa, N.; Banuelos, O.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Zarzuelo, A.; Utrilla, M.P. Intestinal anti-inflammatory activity of hydroalcoholic extracts of P. purpureal and P. lychnitisl. In the trinitrobenzenesulphonic acid model of rat colitis. J. Ethnopharmacol. 2013, 146, 750–759. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [Green Version]
- Caliş, I.; Kirmizibekmez, H. Glycosides from Phlomis lunariifolia. Phytochemistry 2004, 65, 2619–2625. [Google Scholar] [CrossRef]
- Li, M.; Shang, X.; Jia, Z.; Zhang, R. Biodiversity Phytochemical and biological studies of plants from the genus Phlomis. Chem. Biodivers. 2010, 7, 283–301. [Google Scholar] [CrossRef]






| Normal Diet | |
|---|---|
| Sucrose | 01 |
| Ash | 5% |
| Fat | 6% |
| Crude protein | 18% |
| Crude fibre | 5.5 |
| Nitrogen free extract (NFE) | 64.5% |
| Major LC/MS m/z (Intensity) | Molecular for-Molar Mass Mula | Name of Identified Compound | ||
|---|---|---|---|---|
| 1.73 | 169 (90), 135 (45). | C8H8O4 | 168 | Vanillic acid |
| 4.00 | 303 (100) 285 (20), 243.082, 178.998, 151.004 | C15H10O | 302 | Querctin |
| 1.85 | 225 (30), 208 (100), 164 (20). | C11H12O5 | 224 | Sinapinic acid |
| Positive Control | Negative Control | Treatment Group | Kruskal Wall Test for Histology Asymptotic Significance Level (p < 0.05) | |
|---|---|---|---|---|
| Sinusoidal dilatation | 00.25 ± 00.11 | 1.44 ± 00.12 | 0.83 ± 0.02 | 0.005 |
| Focal necrosis | 00.16 ± 00.09 | 1.51 ± 00.08 | 0.91 ± 0.15 | 0.001 |
| Pyknotic nuclei | 00.53 ± 00.01 | 1.53 ± 00.01 | 0.92 ± 0.12 | 0.002 |
| Lobular necrosis (Central) | 00.16 ± 00.08 | 1.81 ± 00.0 | 0.92 ± 0.18 | 0.001 |
| Eccentric nuclei at hepatocytes | 00.24 ± 00.07 | 2.01 ± 00.17 | 1.0 ± 0.22 | 0.001 |
| Cytoplasmic vacuolation of tubular epithelium | 00.33 ± 0.09 | 1.51 ± 0.02 | 00.76 ± 0.11 | 0.01 |
| Positive Control | Negative Control | Treatment Group | Kruskal Wall Test for Histology Asymptotic Significance Level (p < 0.05) | |
|---|---|---|---|---|
| Renal Tubular thickening | 00.166 ± 0.10 | 1.51 ± 00.006 | 1.07 ± 00.10 | 0.002 |
| Infiltration of Interstitial | 00.165 ± 0.10 | 1.76 ± 00.07 | 00.90 ± 00.15 | 0.004 |
| Cytoplasmic vacuolation at renal tubular epithelium | 00.24 ± 0.07 | 1.92 ± 00.08 | 00.84 ± 00.15 | 0.011 |
| Renal Tubular necrosis | 00.25 ± 0.07 | 1.59 ± 00.22 | 1.0 ± 00.11 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, M.U.; Naqvi, S.A.R.; Rasool, N.; Shah, S.A.A.; Zakaria, Z.A. Anti-Diabetic and Cytotoxic Evaluation of Phlomis stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats. Metabolites 2022, 12, 1133. https://doi.org/10.3390/metabo12111133
Rasheed MU, Naqvi SAR, Rasool N, Shah SAA, Zakaria ZA. Anti-Diabetic and Cytotoxic Evaluation of Phlomis stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats. Metabolites. 2022; 12(11):1133. https://doi.org/10.3390/metabo12111133
Chicago/Turabian StyleRasheed, Mamoon Ur, Syed Ali Raza Naqvi, Nasir Rasool, Syed Adnan Ali Shah, and Zainul Amiruddin Zakaria. 2022. "Anti-Diabetic and Cytotoxic Evaluation of Phlomis stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats" Metabolites 12, no. 11: 1133. https://doi.org/10.3390/metabo12111133
APA StyleRasheed, M. U., Naqvi, S. A. R., Rasool, N., Shah, S. A. A., & Zakaria, Z. A. (2022). Anti-Diabetic and Cytotoxic Evaluation of Phlomis stewartii Plant Phytochemicals on Cigarette Smoke Inhalation and Alloxan-Induced Diabetes in Wistar Rats. Metabolites, 12(11), 1133. https://doi.org/10.3390/metabo12111133