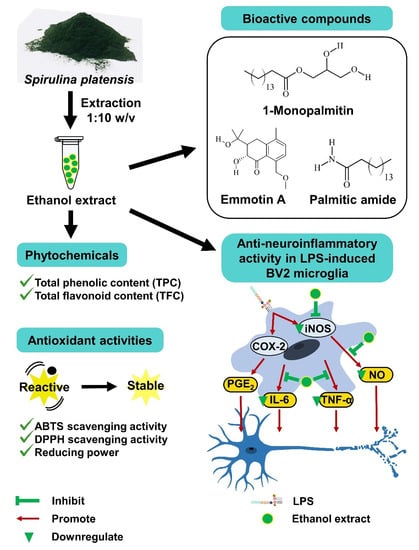
Spirulina platensis Suppressed iNOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Solvent Extracts Preparation
2.3. Phytochemical Screening
2.3.1. Total Phenolic Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.4. Antioxidant Capacity
2.4.1. ABTS Scavenging Activity
2.4.2. DPPH Scavenging Activity
2.4.3. Reducing Power
2.5. Cell Culture
2.6. Cell Viability
2.7. Anti-Neuroinflammatory Activity
2.7.1. Griess Assay
2.7.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7.3. Western Blot Analysis
2.8. Bioactive Compounds Identification
2.9. Statistical Analysis
3. Results
3.1. Yield Percentage and Phytochemical Content
3.2. Antioxidant Capacity of Solvent Extracts
3.3. Effect of Solvent Extracts on Cell Viability
3.4. Solvent Extracts Inhibited LPS-Induced Production of NO
3.5. Ethanol Extract Inhibited LPS-Induced Production of PGE2, TNF-α, and IL-6
3.6. Ethanol Extract Downregulated LPS-Induced Expression of iNOS but Upregulated COX-2
3.7. Bioactive Compounds Profile of Ethanol Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 20 August 2022).
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Brown, G.C.; Vilalta, A. How microglia kill neurons. Brain Res. 2015, 1628 Pt B, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wen, L.L.; Huang, Y.N.; Chen, Y.T.; Ku, M.C. Dual effects of antioxidants in neurodegeneration: Direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006, 12, 3521–3533. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, P38 and the NF-κB signaling pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanaka, T.; Okochi, M. New drugs for Alzheimer’s disease in Japan. Psychiatry Clin. Neurosci. 2011, 65, 399–404. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Medications for Memory, Cognition and Dementia-Related Behaviors. Available online: https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory (accessed on 20 August 2022).
- Subermaniam, K.; Teoh, S.L.; Yow, Y.Y.; Tang, Y.Q.; Lim, L.W.; Wong, K.H. Marine algae as emerging therapeutic alternatives for depression: A review. Iran J. Basic Med. Sci. 2021, 24, 997–1013. [Google Scholar] [CrossRef]
- Pang, J.R.; Goh, V.M.J.; Tan, C.Y.; Phang, S.M.; Wong, K.H.; Yow, Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018, 30, 3253–3260. [Google Scholar] [CrossRef]
- Pang, J.R.; How, S.W.; Wong, K.H.; Lim, S.H.; Phang, S.M.; Yow, Y.Y. Cholinesterase inhibitory activities of neuroprotective fraction derived from red alga Gracilaria manilaensis. Fish Aquat. Sci. 2022, 25, 49–63. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium oligomannate: First approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Balachandran, P.; Pugh, N.D.; Ma, G.; Pasco, D.S. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice. Int. Immunopharmacol. 2006, 6, 1808–1814. [Google Scholar] [CrossRef]
- Akbarizare, M.; Ofoghi, H.; Hadizadeh, M.; Moazami, N. In vitro assessment of the cytotoxic effects of secondary metabolites from Spirulina platensis on hepatocellular carcinoma. Egypt Liver J. 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Kok, Y.Y.; Chu, W.L.; Phang, S.M.; Mohamed, S.M.; Naidu, R.; Lai, P.J.; Ling, S.N.; Mak, J.W.; Lim, P.K.C.; Balraj, P.; et al. Inhibitory activities of microalgal extracts against Epstein-Barr virus DNA release from lymphoblastoid cells. J. Zhejiang Univ. Sci. B 2011, 12, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Asker, M.M.S.; Ibrahim, Z.K. Functional bioactive compounds and biological activities of Spirulina platensis lipids. Czech J. Food Sci. 2008, 26, 211–222. [Google Scholar] [CrossRef]
- Mallikarjun, G.K.G.; Udaya, S.K.; Sarada, R.; Ravishankar, G.A. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J. Food Sci. Technol. 2015, 52, 3627–3633. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.G.; Hou, C.W.; Lee, S.Y.; Chuang, Y.; Lin, C.C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab. Syndr. Obes. 2018, 11, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; Viana, G.S.B. Neuroprotective activities of Spirulina platensis in the 6-OHDA model of Parkinson’s disease are related to its anti-Inflammatory effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, J.; Zhang, J.G.; Xie, J.X. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural Regen. Res. 2015, 10, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ngu, E.L.; Ko, C.L.; Tan, C.Y.; Wong, K.H.; Phang, S.M.; Yow, Y.Y. Phytochemical profiling and in vitro screening for neuritogenic and antioxidant activities of Spirulina platensis. Indian J. Pharm. Educ. Res. 2021, 55, 812–822. [Google Scholar] [CrossRef]
- Aziz, I.; Ramli, M.D.C.; Zain, N.S.M.; Sanusi, J. Behavioral and histopathological study of changes in spinal cord injured rats supplemented with Spirulina platensis. Evid. Based Complement Alternat. Med. 2014, 2014, 871657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Lo, T.S.; Hammer, K.D.P.; Zegarra, M.; Cho, W.C.S. Methenamine: A forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev. Anti. Infect. Ther. 2014, 12, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Ozpinar, A.; Ozpinar, A.; Perez, J.L.; Elmaci, İ. Methenamine’s journey of 160 years: Repurposal of an old urinary antiseptic for treatment and hypoxic radiosensitization of cancers and glioblastoma. Clin. Exp. Pharmacol. Physiol. 2019, 46, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegmund, B.; Balser, C.; Ladilov, Y.V.; Piper, H.M. Protection of isolated cardiomyocytes against reoxygenation-induced hypercontracture by SIN-1C. Basic Res. Cardiol. 1998, 93 (Suppl. 3), 17–20. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Yong, Q.C.; Seqqat, R.; Chandel, N.; Feldman, D.L.; Baker, K.M.; Kumar, R. Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: Comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clin. Sci. 2013, 124, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.L.; Barclay, B.W.; Navarrete, A.E.; Wosu, N.J.; Sahota, P. Protective effects of valsartan and benazeprilat in salt-loaded stroke- prone spontaneously hypertensive rats. Clin. Exp. Hypertens. 1998, 20, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Castor, M.G.M.; Santos, R.A.S.; Duarte, I.D.G.; Romero, T.R.L. Angiotensin-(1-7) through Mas receptor activation induces peripheral antinociception by interaction with adrenoreceptors. Peptides 2015, 69, 80–85. [Google Scholar] [CrossRef]
- Ok, S.H.; Kwon, S.C.; Baik, J.; Hong, J.M.; Oh, J.; Han, J.Y.; Sohn, J.T. Dexmedetomidine-induced contraction involves CPI-17 phosphorylation in isolated rat aortas. Int. J. Mol. Sci. 2016, 17, 1663. [Google Scholar] [CrossRef] [Green Version]
- Sargent, C.A.; Dzwonczyk, S.; Grover, G.J. The effect of α2-adrenoceptor antagonists in isolated globally ischemic rat hearts. Eur. J. Pharmacol. 1994, 261, 25–32. [Google Scholar] [CrossRef]
- Alachkar, A.; Brotchie, J.M.; Jones, O.T. Locomotor response to L-DOPA in reserpine-treated rats following central inhibition of aromatic L-amino acid decarboxylase: Further evidence for non-dopaminergic actions of L-DOPA and its metabolites. Neurosci. Res. 2010, 68, 44–50. [Google Scholar] [CrossRef]
- Gupta, S.; Khanna, V.K.; Maurya, A.; Bawankule, D.U.; Shukla, R.K.; Pal, A.; Srivastava, S.K. Bioactivity guided isolation of antipsychotic constituents from the leaves of Rauwolfia tetraphylla L. Fitoterapia 2012, 83, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef] [Green Version]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Molina-Jasso, D.; Reyes-Cadena, S.; Álvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, J. Antigenotoxic, antioxidant and lymphocyte induction effects produced by pteropodine. Basic Clin. Pharmacol. Toxicol. 2009, 104, 222–227. [Google Scholar] [CrossRef]
- Rinner, B.; Li, Z.X.; Haas, H.; Siegl, V.; Sturm, S.; Stuppner, H.; Pfragner, R. Antiproliferative and pro-apoptotic effects of Uncaria tomentosa in human medullary thyroid carcinoma cells. Anticancer Res. 2009, 29, 4519–4528. [Google Scholar] [PubMed]
- Kaiser, S.; Dietrich, F.; de Resende, P.E.; Verza, S.G.; Moraes, R.C.; Morrone, F.B.; Batastini, A.M.O.; Ortega, G.G. Cat’s claw oxindole alkaloid isomerization induced by cell incubation and cytotoxic activity against T24 and RT4 human bladder cancer cell lines. Planta Med. 2013, 79, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef]
- Pulcinelli, F.M.; Riondino, S.; Celestini, A.; Pignatelli, P.; Trifirò, E.; Di Renzo, L.; Violi, F. Persistent production of platelet thromboxane A2 in patients chronically treated with aspirin. J. Thromb. Haemost. 2005, 3, 2784–2789. [Google Scholar] [CrossRef]
- Heffner, J.E.; Shoemaker, S.A.; Canham, E.M.; Patel, M.; McMurtry, I.F.; Morris, H.G.; Repine, J.E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A. J. Clin. Investig. 1983, 71, 351–357. [Google Scholar] [CrossRef]
- Cook, J.A.; Wise, W.C.; Halushka, P.V. Elevated thromboxane levels in the rat during endotoxic shock. Protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. J. Clin. Investig. 1980, 65, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Saleem, H.; Sarfraz, M.; Khan, K.M.; Anwar, M.A.; Zengin, G.; Ahmad, I.; Khan, S.; Mahomoodally, M.F.; Ahemad, N. UHPLC-MS phytochemical profiling, biological propensities and in-silico studies of Alhagi maurorum roots: A medicinal herb with multifunctional properties. Drug Dev. Ind. Pharm. 2020, 46, 861–868. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Schevenels, F.T. A novel cyclohexenone from Trachyspermum roxburghianum. Nat. Prod. Res. 2018, 32, 2499–2504. [Google Scholar] [CrossRef]
- Boopathi, S.; Vashisth, R.; Manoharan, P.; Kandasamy, R.; Sivakumar, N. Stigmatellin Y–An anti-biofilm compound from Bacillus subtilis BR4 possibly interferes in PQS–PqsR mediated quorum sensing system in Pseudomonas aeruginosa. Bioorganic Med. Chem. Lett. 2017, 27, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Han, J.Y.; Lee, S.H.; Jee, S.H.; Lee, J.H. The metabolites in peripheral blood mononuclear cells showed greater differences between patients with impaired fasting glucose or type 2 diabetes and healthy controls than those in plasma. Diabetes Vasc. Dis. Res. 2017, 14, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Deng, D.; Chen, H.; Cheng, W.; Li, Q.; Luo, R.; Ding, S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015, 407, 4683–4695. [Google Scholar] [CrossRef]
- Li, S.S.; Liu, Y.; Li, H.; Wang, L.P.; Xue, L.F.; Yin, G.S.; Wu, X.S. Identification of psoriasis vulgaris biomarkers in human plasma by non-targeted metabolomics based on UPLC-Q-TOF/MS. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3940–3950. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Wang, M.; Liu, L.; Sun, X.; Ma, L.; Xie, W.; Wang, C.; Tang, S.; Wang, D.; et al. Lysophosphatidylcholine and amide as metabolites for detecting Alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J. Neuropathol. Exp. Neurol. 2014, 73, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Dabur, R.; Mittal, A. Detection and qualitative analysis of fatty acid amides in the urine of alcoholics using HPLC-QTOF-MS. Alcohol 2016, 52, 71–78. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, M.; Jana, M.; Mishra, R.K.; Yung, Y.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. Identification and characterization of PPARα ligands in the hippocampus. Nat. Chem. Biol. 2016, 12, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Yusoff, N.I.N.; Uzir, B.F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-glucosidase inhibitors from Clinacanthus nutans lindau leaves by gas chromatography-mass spectrometry-based metabolomics and molecular docking simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Jeong, I.H.; Kong, B.S.; Lee, J.E.; Kim, K.H.; Lee, D.Y.; Kim, H.J. Disease type- and status-specific alteration of CSF metabolome coordinated with clinical parameters in inflammatory demyelinating diseases of CNS. PLoS ONE 2016, 11, e0166277. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Zhu, P.; Pi, F.; Sun, C.; Jiang, H.; Sun, J.; Wang, X.; Zhang, Y.; Sun, X. GC-TOF/MS-based metabolomic strategy for combined toxicity effects of deoxynivalenol and zearalenone on murine macrophage ANA-1 cells. Toxicon 2016, 120, 175–184. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, Y.; Chen, M.; Tan, N.; Liu, S.; Nie, X.; Zhou, W. Serum metabolomics reveals metabolic profiling for women with hyperandrogenism and insulin resistance in polycystic ovary syndrome. Metabolomics 2020, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Contaifer, D., Jr.; Roberts, C.H.; Kumar, N.G.; Natarajan, R.; Fisher, B.J.; Leslie, K.; Reed, J.; Toor, A.A.; Wijesinghe, D.S. A preliminary investigation towards the risk stratification of allogeneic stem cell recipients with respect to the potential for development of GVHD via their pre-transplant plasma lipid and metabolic signature. Cancers 2019, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghe, A.; Ramanujam, V.M.S.; Phillips, J.D.; Desnick, R.J.; Anderson, K.E. Harderoporphyria: Case of lifelong photosensitivity associated with compound heterozygous coproporphyrinogen oxidase (CPOX) mutations. Mol. Genet. Metab. Rep. 2019, 19, 100457. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.J.C.; Schmitt, J.D.; Wykle, R.L.; Daniel, L.W. 1-O-hexadecyl-2-acetyl-sn-glycerol stimulates differentiation of HL-60 human promyelocytic leukemia cells to macrophage-like cells. Biochem. Biophys. Res. Commun. 1984, 122, 824–830. [Google Scholar] [CrossRef]
- Holly, S.P.; Gera, N.; Wang, P.; Wilson, A.; Guan, Z.; Lin, L.; Cooley, B.; Alfar, H.R.; Patil, R.G.; Piatt, R.; et al. Ether lipid metabolism by AADACL1 regulates platelet function and thrombosis. Blood Adv. 2019, 3, 3818–3828. [Google Scholar] [CrossRef] [Green Version]
- Slater, S.J.; Seiz, J.L.; Stagliano, B.A.; Cook, A.C.; Milano, S.K.; Ho, C.; Stubbs, C.D. Low- and high-affinity phorbol ester and diglyceride interactions with protein kinase C: 1-O-alkyl-2-acyl-sn-glycerol enhances phorbol ester- and diacylglycerol-induced activity but alone does not induce activity. Biochemistry 2001, 40, 6085–6092. [Google Scholar] [CrossRef]
- Prinville, V.; Ohlund, L.; Sleno, L. Targeted analysis of 46 bile acids to study the effect of acetaminophen in rat by LC-MS/MS. Metabolites 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.M.A.P.; Messias, M.C.F.; Duarte, G.H.B.; de Santis, G.K.D.; Mecatti, G.C.; Porcari, A.M.; Murgu, M.; Simionato, A.V.C.; Rocha, T.; Martinez, C.A.R.; et al. Plasma lipid profile reveals plasmalogens as potential biomarkers for colon cancer screening. Metabolites 2020, 10, 262. [Google Scholar] [CrossRef]
- Forestrania, R.C.; Anaya-Eugenio, G.D.; Acuña, U.M.; Ren, Y.; Elya, B.; de Blanco, E.C. Secondary metabolites from Garcinia daedalanthera Pierre leaves (Clusiaceae). Nat. Prod. Res. 2020, 36, 207–213. [Google Scholar] [CrossRef]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Khan, N.A. Antibacterial activities of selected pure compounds isolated from gut bacteria of animals living in polluted environments. Antibiotics 2020, 9, 190. [Google Scholar] [CrossRef]
- Sanders, R.J.; Ofman, R.; Dekker, C.; Kemp, S.; Wanders, R.J.A. Enzymatic diagnosis of Sjögren-Larsson syndrome using electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 877, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lin, S.; Cai, Z. Fatty acid profiles reveal toxic responses in adipose tissue of C57BL/6J mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal. Methods 2014, 6, 8207–8211. [Google Scholar] [CrossRef]
- Boelaert, J.; t’Kindt, R.; Schepers, E.; Jorge, L.; Glorieux, G.; Neirynck, N.; Lynen, F.; Sandra, P.; Vanholder, R.; Sandra, K. State-of-the-art non-targeted metabolomics in the study of chronic kidney disease. Metabolomics 2014, 10, 425–442. [Google Scholar] [CrossRef]
- Heazell, A.E.P.; Bernatavicius, G.; Warrander, L.; Brown, M.C.; Dunn, W.B. A metabolomic approach identifies differences in maternal serum in third trimester pregnancies that end in poor perinatal outcome. Reprod. Sci. 2012, 19, 863–875. [Google Scholar] [CrossRef]
- Chew, Y.L.; Goh, J.K.; Lim, Y.Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009, 116, 13–18. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical changes of bioactive phytochemicals during thermal processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–9. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Gargouri, M.; Magné, C.; Feki, A.E. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr. Res. 2016, 36, 1255–1268. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; Paepe, D.D.; Baart, G.J.E.; Cooman, L.D. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Klejdus, B.; Kopecký, J.; Benešová, L.; Vacek, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A 2009, 1216, 763–771. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J. Food Qual. 2019, 2019, 3707219. [Google Scholar] [CrossRef] [Green Version]
- Bellahcen, T.O.; Aamiri, A.; Touam, I.; Hmimid, F.; Amrani, A.E.; Cherif, A.; Cherki, M. Evaluation of Moroccan microalgae: Spirulina platensis as a potential source of natural antioxidants. J. Complement Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Herrero, M.; Soler-Rivas, C.; Santoyo, S.; Señorans, F.J.; Cifuentes, A.; Ibañez, E. Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 2005, 28, 2111–2119. [Google Scholar] [CrossRef] [Green Version]
- Subermaniam, K.; Yow, Y.Y.; Lim, S.H.; Koh, O.H.; Wong, K.H. Malaysian macroalga Padina australis Hauck attenuates high dose corticosterone-mediated oxidative damage in PC12 cells mimicking the effects of depression. Saudi J. Biol. Sci. 2020, 27, 1435–1445. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Marín, F.R.; Hernández, S.F.; Arredondo, B.O.; Señoráns, F.J.; Ibañez, E.; Reglero, G. Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J. Sep. Sci. 2005, 28, 1031–1038. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Hunot, S.; Dugas, N.; Faucheux, B.; Hartmann, A.; Tardieu, M.; Debré, P.; Agid, Y.; Dugas, B.; Hirsch, E.C. FcεRII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J. Neurosci. 1999, 19, 3440–3447. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s Disease. J. Neuroimmunol. 2016, 291, 115–124. [Google Scholar] [CrossRef]
- Moncada, S.; Bolaños, J.P. Nitric oxide, cell bioenergetics and neurodegeneration. J. Neurochem. 2006, 97, 1676–1689. [Google Scholar] [CrossRef]
- Benarroch, E.E. Nitric oxide: A pleiotropic signal in the nervous system. Neurology 2011, 77, 1568–1576. [Google Scholar] [CrossRef]
- Yin, L.; Xie, Y.; Yin, S.; Lv, X.; Zhang, J.; Gu, Z.; Sun, H.; Liu, S. The S-nitrosylation status of PCNA localized in cytosol impacts the apoptotic pathway in a Parkinson’s disease paradigm. PLoS ONE 2015, 10, e0117546. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.L.; Jones, F.; Kubota, E.S.F.C.S.; Pocock, J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef] [Green Version]
- Piovan, A.; Battaglia, J.; Filippini, R.; Costa, V.D.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre- and early post-treatment with Arthrospira platensis (Spirulina) extract impedes lipopolysaccharide-triggered neuroinflammation in microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Mohsen, G.A.-M.; Antonisamy, P.; Arokiyaraj, S.; Kim, H.-J.; Kim, S.-J.; Park, K.H.; Kim, Y.O. Spirulina consumption effectively reduces anti-inflammatory and pain related infectious diseases. J. Infect. Public Health 2019, 12, 777–782. [Google Scholar] [CrossRef]
- Aïd, S.; Bosetti, F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Liu, K.S.; Yang, T.J.; Hwang, J.H.; Chan, Y.C.; Lee, I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef]
- Choi, W.Y.; Sim, J.H.; Lee, J.Y.; Kang, D.H.; Lee, H.Y. Increased anti-inflammatory effects on LPS-induced microglia cells by Spirulina maxima extract from ultrasonic process. Appl. Sci. 2019, 9, 2144. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.W.; Chen, F. Fucoxanthinol from the diatom Nitzschia laevis ameliorates neuroinflammatory responses in lipopolysaccharide-stimulated BV-2 microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piovan, A.; Filippini, R.; Corbioli, G.; Costa, V.D.; Giunco, E.M.V.; Burbello, G.; Pagetta, A.; Giusti, P.; Zusso, M. Carotenoid extract derived from Euglena gracilis overcomes lipopolysaccharide-induced neuroinflammation in microglia: Role of NFκB and Nrf2 signaling pathways. Mol. Neurobiol. 2021, 58, 3515–3528. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-kulesza, K.A.; Targowska-duda, K.; Klimek, K.; Ginalska, G.; Jozwiak, K.; Waksmundzka-Hajnos, M.; Ciesla, L.M. Volatile terpenoids as potential drug leads in Alzheimer’ s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Shen, L.; Chen, Z.; Xu, L.; Zhang, J.; Yu, X. Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 2016, 1649 Pt A, 30–37. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-Neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Patel, M.; Ashraf, S.A.; Jamal, A.; Awadelkareem, A.M.; Sachidanandan, M.; Snoussi, M.; Feo, V.D. Phytochemistry, bioactivities, pharmacokinetics and toxicity prediction of Selaginella repanda with its anticancer potential against human lung, breast and colorectal carcinoma cell lines. Molecules 2021, 26, 768. [Google Scholar] [CrossRef]
- Lai, N.J.Y.; Ngu, E.L.; Pang, J.R.; Wong, K.H.; Ardianto, C.; Ming, L.C.; Lim, S.H.; Walvekar, S.G.; Anwar, A.; Yow, Y.Y. Carrageenophyte Kappaphycus malesianus inhibits microglia-mediated neuroinflammation via suppression of AKT/NF-κB and ERK signaling pathways. Mar. Drugs 2022, 20, 534. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.I.; Choi, J.W.; Kim, M.; Yoon, N.Y.; Choi, C.G.; Choi, J.S.; Kim, H.R. Anti-inflammatory effect of hexane fraction from Myagropsis myagroides ethanolic extract in lipopolysaccharide-stimulated BV-2 microglial cells. J. Pharm. Pharmacol. 2013, 65, 895–906. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; Osborne, J.A.; Melanson, R.; Oleary, S.J.B. Nitric oxide inhibitory activity of monogalactosylmonoacylglycerols from a freshwater microalgae Chlorella sorokiniana. Nat. Prod. Res. 2013, 27, 1028–1031. [Google Scholar] [CrossRef]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; Oleary, S.J.B. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]










| Extract | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|---|
| Hexane | 2.98 ± 0.442 a | 4.63 ± 0.594 a | 0.00 ± 0.000 a |
| Ethyl acetate | 5.31 ± 0.766 a | 10.26 ± 0.864 b | 0.29 ± 0.191 a |
| Ethanol | 3.45 ± 0.042 a | 27.35 ± 0.400 c | 83.41 ± 2.049 b |
| Aqueous | 27.75 ± 0.877 b | 32.40 ± 0.515 d | 7.24 ± 0.389 c |
| Extract/Control | EC50 (mg/mL) | ||
|---|---|---|---|
| ABTS | DPPH | Reducing Power | |
| Extract | |||
| Hexane | 0.692 ± 0.02157 a | 0.175 ± 0.00202 a | 1.683 ± 0.02121 a |
| Ethyl acetate | 0.206 ± 0.00060 b | 0.052 ± 0.00350 b | 1.533 ± 0.01966 b |
| Ethanol | 0.097 ± 0.00035 c | 0.107 ± 0.00598 c | 0.8078 ± 0.03707 c |
| Aqueous | 0.305 ± 0.01583 d | 0.367 ± 0.01493 d | 1.025 ± 0.01155 d |
| Positive control | |||
| Ascorbic acid | 0.009 ± 0.00013 e | 0.001 ± 0.00002 e | 0.008 ± 0.00047 e |
| No. | Compound Name | Molecular Formula | Molecular Mass | m/z Ratio [Ion] | Molecular Structure | Classification | Bioactivity |
|---|---|---|---|---|---|---|---|
| i | Methenamine | C6H12N4 | 140.106 | 141.1132 [M + H]+ |  | Amines | FDA-approved antiseptic agent to prevent recurrent urinary tract infections [33]. Anticancer activity in systemic cancers and glioblastoma [34]. |
| ii | (Morpholinoimino)acetonitrile | C6H9N3O | 139.0742 | 157.1081 [M + NH4]+ |  | Nitriles | Protect against ischemia-reperfusion injury [35]. |
| iii | Benazeprilat | C22H24N2O5 | 396.1687 | 397.1761 [M + H]+ |  | Dipeptide | An ACEi that protects against diabetic cardiomyopathy [36] and hypertension [37]. |
| iv | Rauwolscine | C21H26N2O3 | 354.1938 | 355.2013 [M + H]+ |  | Alkaloid | An α2C-adrenoceptor antagonist that protects against peripheral antinociception [38], hypertension [39], myocardial ischemia [40], hyperactivity [41], psychosis [42] and breast cancer [43]. |
| v | Uncarine C | C21H24N2O4 | 368.1734 | 369.1806 [M + H]+ |  | Alkaloid | Antigenotoxic, antioxidant, and immunomodulatory activity [44]. Anticancer activity in medullary thyroid cancer [45], bladder cancer [46], and lymphoblastic leukemia [47]. |
| vi | 2-Carboxy-4-dodecanolide | C13H22O4 | 242.152 | 243.1593 [M + H]+ |  | γ-butyrolactone | N/A |
| vii | 4,5-Di-O-methyl-8-prenylafzelechin-4beta-ol | C22H26O6 | 386.1734 | 404.2063 [M + NH4]+ |  | Flavonoid | N/A |
| viii | (±)13-Azaprostanoic acid | C19H37NO2 | 311.2822 | 329.3163 [M + NH4]+ |  | Fatty acid | Antagonist of thromboxane/endoperoxide receptor that protects against thrombosis [48], hypertension [49], and endotoxic shock [50]. |
| ix | Estra-1,3,5(10)-triene-2,17beta-diol | C18H24O2 | 272.1774 | 273.1848 [M + H]+ |  | Terpenoid | N/A |
| x | 15(S)-15-methyl PGF2α ethyl amide | C23H41NO4 | 395.3029 | 396.3106 [M + H]+ |  | N/A | N/A |
| xi | Emmotin A | C16H22O4 | 278.1521 | 279.1594 [M + H]+ |  | Terpenoid | An enzyme inhibitor that has binding interaction with AChE, BChE, α-glucosidase, α-amylase, and tyrosine [51]. |
| xii | 3-Butylidene-7-hydroxyphthalide | C12H12O3 | 204.0786 | 205.0858 [M + H]+ |  | Phthalide | Anticancer activity in human small cell lung cancer [52]. |
| xiii | N-cis-tetradec-9Z-enoyl-L-Homoserine lactone | C18H31NO3 | 309.2303 | 310.2374 [M + H]+ |  | Lactone | N/A |
| xiv | Stigmatellin Y | C29H40O6 | 484.2826 | 502.3166 [M + NH4]+ |  | Phenolic | Inhibit the virulence of Pseudomonas aeruginosa [53]. |
| xv | Palmitic amide | C16H33NO | 255.2562 | 256.2636 [M + H]+ |  | Fatty acid amide | Biomarker for diabetes [54], PCOS [55], psoriasis vulgaris [56], AD [57] and alcoholism [58]. A ligand of PPARα that upregulates synaptic function in hippocampal neurons [59]. |
| xvi | 1-monopalmitin | C19H38O4 | 330.2773 | 353.2669 [M + Na]+ |  | Glycerolipid | Antidiabetic activity [60]. Biomarker for CNS iDDs [61], mycotoxins exposure [62], PCOS with complications [63], and stem cell transplantation recipients [64]. |
| xvii | Harderoporphyrin | C35H36N4O6 | 608.2636 | 609.2708 [M + H]+ |  | Porphyrin | Biomarker for harderoporphyria [65]. |
| xviii | Hexadecyl acetyl glycerol | C21H42O4 | 358.3092 | 381.2983 [M + Na]+ |  | Ether | Induce differentiation in human promyelocytic leukemia cell line HL-60 [66]. Inhibit platelet aggregation [67]. Modulate PKC activity [68]. |
| xix | 3α,12α-Dihydroxy-5β-chol-8(14)-en-24-oic Acid | C24H38O4 | 390.278 | 391.2854 [M + H]+ |  | Terpenoid | Biomarker for acetaminophen-related toxicity [69] and colon cancer [70]. |
| xx | Docosanedioic acid | C22H42O4 | 370.3077 | 371.3151 [M + H]+ |  | Fatty acid | Antioxidant activity in human HT-29 colon cancer cells [71]. Bactericidal activity against P. aeruginosa [72]. Biomarker for Sjogren–Larsson syndrome [73] and patients exposed to TCDD [74]. |
| xxi | Hexacosanedioic acid | C26H50O4 | 426.3705 | 449.3598 [M + Na]+ |  | Fatty acid | Biomarker for chronic kidney disorder [75] and poor pregnancy prediction [76]. |
| No. | Molecular Formula | Molecular Mass | m/z Ratio | Ion |
|---|---|---|---|---|
| i | C36H66N6O6 | 678.5048 | 679.5121 | [M + H]+ |
| ii | C13H20O4 | 240.1365 | 241.1435 | [M + H]+ |
| iii | C9H19NO | 157.1463 | 158.1536 | [M + H]+ |
| iv | C8H4O3 | 148.0157 | 149.023 | [M + H]+ |
| v | C18H38O4 | 318.2766 | 336.3108 | [M + NH4]+ |
| vi | C16H34O3 | 274.2506 | 275.258 | [M + H]+ |
| vii | C37H74N2O7S | 690.5213 | 691.5291 | [M + H]+ |
| viii | C18H38O3 | 302.2813 | 325.2736 | [M + Na]+ |
| ix | C35H42O10 | 622.2766 | 623.2835 | [M + H]+ |
| x | C36H38N4O5 | 606.2846 | 607.2916 | [M + H]+ |
| xi | C37H40N4O5 | 620.2998 | 621.3072 | [M + H]+ |
| xii | C38H36N8O | 620.3005 | 621.3076 | [M + H]+ |
| xiii | C38H51N3O | 565.4037 | 566.4108 | [M + H]+ |
| xiv | C44H58N2O3 | 662.4455 | 663.4537 | [M + H]+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngu, E.-L.; Tan, C.-Y.; Lai, N.J.-Y.; Wong, K.-H.; Lim, S.-H.; Ming, L.C.; Tan, K.-O.; Phang, S.-M.; Yow, Y.-Y. Spirulina platensis Suppressed iNOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia. Metabolites 2022, 12, 1147. https://doi.org/10.3390/metabo12111147
Ngu E-L, Tan C-Y, Lai NJ-Y, Wong K-H, Lim S-H, Ming LC, Tan K-O, Phang S-M, Yow Y-Y. Spirulina platensis Suppressed iNOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia. Metabolites. 2022; 12(11):1147. https://doi.org/10.3390/metabo12111147
Chicago/Turabian StyleNgu, Ee-Ling, Cheng-Yau Tan, Nicole Jean-Yean Lai, Kah-Hui Wong, Siew-Huah Lim, Long Chiau Ming, Kuan-Onn Tan, Siew-Moi Phang, and Yoon-Yen Yow. 2022. "Spirulina platensis Suppressed iNOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia" Metabolites 12, no. 11: 1147. https://doi.org/10.3390/metabo12111147
APA StyleNgu, E. -L., Tan, C. -Y., Lai, N. J. -Y., Wong, K. -H., Lim, S. -H., Ming, L. C., Tan, K. -O., Phang, S. -M., & Yow, Y. -Y. (2022). Spirulina platensis Suppressed iNOS and Proinflammatory Cytokines in Lipopolysaccharide-Induced BV2 Microglia. Metabolites, 12(11), 1147. https://doi.org/10.3390/metabo12111147