Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov
Abstract
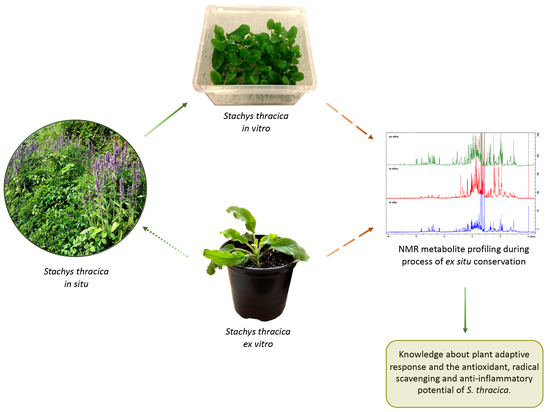
:1. Introduction
2. Results and Discussion
2.1. In Vitro Cultivation and Ex Vitro Acclimation of S. thracica
2.2. Genetic Stability of In Vitro Cultivated and Ex Vitro Adapted S. thracica
2.3. NMR-Based Metabolite Profiling during S. thracica Ex Situ Conservation
2.4. Comparative Determination of Total Phenols and Flavonoids in In Situ, In Vitro Cultivated, and Ex Vitro Adapted Plants
2.5. Antioxidant and Radical Scavenging Activity of S. thracica during the Process of Ex Situ Conservation
2.6. Anti-Inflammatory Activity of S. thracica
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Culture Conditions
3.3. Ex Vitro Acclimation
3.4. Genetic Stability Assay by SRAP Markers
3.5. Extraction Procedure and NMR Analyses
3.6. Methanolic Extract Preparation
3.7. Determination of Total Phenolic and Flavonoid Content
3.8. Total Antioxidant Activity
3.9. DPPH Radical Scavenging Activity
3.10. ABTS Radical Scavenging Activity
3.11. FRAP Assay
3.12. Microtitre Hemolytic Complement Assay
3.13. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tomou, E.M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Peev, D.; Vladimirov, V. Red Data Book of the Republic of Bulgaria—Volume 1 Plants and Fungi; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2015; Available online: http://e-ecodb.bas.bg/rdb/en/vol1/ (accessed on 12 March 2022).
- Conforti, F.; Menichini, F.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A. Comparative chemical composition, free radical scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of Mediterranean Area. Food Chem. 2009, 116, 898–905. [Google Scholar] [CrossRef]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Vundać, V.B.; Brantner, A.H.; Plazibat, M. Content of polyphenolic constituents and antioxidant activity of some Stachys taxa. Food Chem. 2007, 104, 1277–1281. [Google Scholar] [CrossRef]
- Háznagy-Radnai, E.; Balogh, A.; Czigle, S.; Máthé, I.; Hohmann, J.; Blazsó, G. Antiinflammatory activities of Hungarian Stachys species and their iridoids. Phytother. Res. 2012, 26, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Kotsos, M.; Aligiannis, N.; Mitaku, S.; Skaltsounis, A.L.; Charvala, C. Chemistry of plants from Crete: Stachyspinoside, a new flavonoid glycoside and iridoids from Stachys spinosa. Nat. Prod. Lett. 2001, 15, 377–386. [Google Scholar] [CrossRef]
- Demirtas, I.; Gecibesler, I.H.; Yaglioglu, A.S. Antiproliferative activities of isolated flavone glycosides and fatty acids from Stachys byzantina. Phytochem. Lett. 2013, 6, 209–214. [Google Scholar] [CrossRef]
- Delazar, A.; Delnavazi, M.R.; Nahar, L.; Moghadam, S.B.; Mojarab, M.; Gupta, A.; Sarker, S.D. Lavandulifolioside B: A new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat. Prod. Res. 2011, 25, 8–16. [Google Scholar] [CrossRef]
- Guo, H.; Saravanakumar, K.; Wang, M.H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal. Agric. Biotechnol. 2018, 15, 235–239. [Google Scholar] [CrossRef]
- Grigorakis, S.; Makris, D.P. Characterisation of polyphenol-containing extracts from Stachys mucronata and evaluation of their antiradical activity. Medicines 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukić, J.; Petrović, S.; Niketić, M. Antioxidant activity of four endemic Stachys taxa. Biol. Pharm. Bull. 2006, 29, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazemiyeh, H.; Shoeb, M.; Movahhedin, N.; Kumarasamy, Y.; Talebpour, A.H.; Delazar, A.; Sarker, S.D. Phenolic compounds and their glycosides from Stachys schtschegleevii (Lamiaceae). Biochem. Syst. Ecol. 2006, 9, 721–723. [Google Scholar] [CrossRef]
- Assyov, B.; Petrova, A. Conspectus of the Bulgarian Vascular Flora. Distribution Maps and Floristic Elements; Boris ASSYOV: Sofia, Bulgaria, 2012; Volume 4. [Google Scholar]
- Bankova, V.; Koeva-Todorovska, J.; Stambolijska, T.; Ignatova-Groceva, M.D.; Todorova, D.; Popov, S. Polyphenols in Stachys and Betonica species (Lamiaceae). Z. Naturforsch. C 1999, 54, 876–880. [Google Scholar] [CrossRef]
- Petrova, A.; Vladimirov, V. Balkan endemics in the Bulgarian flora. Phytol. Balc. 2010, 16, 293–311. [Google Scholar]
- Kapchina-Toteva, V.; Dimitrova, M.A.; Stefanova, M.; Koleva, D.; Kostov, K.; Yordanova, Z.P.; Zhiponova, M.K. Adaptive changes in photosynthetic performance and secondary metabolites during white dead nettle micropropagation. J. Plant Physiol. 2014, 171, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, L.G.; Ivanova, T.A.; Bogdanova, Y.Y.; Gussev, C.V.; Stanilova, M.I.; Bosseva, Y.Z.; Stoeva, T.D. In Vitro cultivation of plant species from sandy dunes along the Bulgarian Black Sea Coast. Phytol. Balc. 2008, 14, 119–123. [Google Scholar]
- Cüce, M.; Bekircan, T.; Laghari, A.H.; Sökmen, M.; Sökmen, A.; Uçar, E.Ö.; Kılıç, A.O. Antioxidant phenolic constituents, antimicrobial and cytotoxic properties of Stachys annua L. from both natural resources and micropropagated plantlets. Indian J. Tradit. Knowl. 2017, 16, 407–416. [Google Scholar]
- Mantovska, D.I.; Kapchina, V.M.; Yordanova, Z.P. In Vitro propagation of the Balkan endemic species Stachys leucoglossa Griseb. Bulg. J. Agric. Sci. 2019, 25, 1211–1215. [Google Scholar]
- Li, G.; Quiros, C. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Zagorcheva, T.; Stanev, S.; Rusanov, K.; Atanassov, I. SRAP markers for genetic diversity assessment of lavender (Lavandula angustifolia Mill.) varieties and breeding lines. Biotechnol. Biotechnol. Equip. 2020, 34, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Alekseeva, M.; Zagorcheva, T.; Rusanova, M.; Rusanov, K.; Atanassov, I. Genetic and flower volatile diversity in natural populations of Origanum vulgare subsp. hirtum (Link) Ietsw. in Bulgaria: Toward the development of a core collection. Front. Plant Sci. 2021, 12, 679063. [Google Scholar] [CrossRef] [PubMed]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crops Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Xiao Hui, Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Yordanova, Z.P.; Rogova, M.A.; Zhiponova, M.K.; Georgiev, M.I.; Kapchina-Toteva, V.M. Comparative determination of the essential oil composition in Bulgarian endemic plant Achillea thracica Velen. during the process of ex situ conservation. Phytochem. Lett. 2017, 20, 456–461. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Hussein, E.; Qouta, L.; Aludatt, M.; Al-Shara, B.; Abu-Zaiton, A. In Vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ. Cult. 2012, 110, 103–110. [Google Scholar] [CrossRef]
- Danova, K.; Nikolova-Damianova, B.; Denev, R.; Dimitrov, D. Influence of vitamins on polyphenolic content, morphological development, and stress response in shoot cultures of Hypericum spp. Plant Cell Tissue Organ. Cult. 2012, 110, 383–393. [Google Scholar] [CrossRef]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Šliumpaite, I.; Venskutonis, P.R.; Murkovic, M.; Ragažinskienė, O. Antioxidant properties and phenolic composition of wood betony (Betonica officinalis L., syn. Stachys officinalis L.). Ind. Crops Prod. 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Maleki-Dizaji, N.; Nazemiyeh, H.; Maddah, N.; Mehmani, F.; Garjani, A. Screening of extracts and fractions from aerial parts of Stachys schtschegleevii Sosn. for anti-inflammatory activities. Pak. J. Pharm. Sci. 2008, 21, 338–343. [Google Scholar]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Ursu, T.M.; Zanfirescu, A.; Radu, G.L. Anti-inflammatory and antioxidant activities of the Impatiens noli-tangere and Stachys officinalis polyphenolic-rich extracts. Rev. Bras. Farmacogn. 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Pastoriza, S.; Delgado-Andrade, C.; Haro, A.; Rufian-Henares, J.A. A physiologic approach to test the global antioxidant response of foods: The GAR method. Food Chem. 2011, 129, 1926–1932. [Google Scholar] [CrossRef]





| Metabolite | S.t. In Situ a | S.t. In Vitro a | S.t. Ex Vitro a | Chemical Shift (ppm) b |
|---|---|---|---|---|
| Amino acids | ||||
| Alanine | + | + | + | δ 1.47 (d, J = 7.2) |
| Valine | + | + | + | δ 0.99 (d, J = 7.0)/δ 1.04 (d, J = 7.0) |
| Sugars | ||||
| α-Glucose | + | + | + | δ 5.17 (d, J = 3.8) |
| β-Glucose | + | + | + | δ 4.56 (d, J = 7.9)/3.18 (dd, J = 7.9, 9.2 ) |
| Sucrose | + | + | ++ | δ 5.37 (d, J = 3.8) |
| Organic acids | ||||
| Acetic acid | + | + | + | δ 1.92 (s) |
| Lactic acid | + | + | + | δ 1.31 (d, J = 6.9)/δ 4.08 m |
| Succinic acid | + | + | + | δ 2.48 (s) |
| Formic acid | + | + | + | δ 8.45 (s) |
| Malic acid | + | + | + | δ 2.80 (dd, J = 16.9, 8.2)/δ 2.93 (dd, J= 16.9, 3.9) |
| Phenolic acids | ||||
| Chlorogenic acid | ++ | ++ | +++ | δ 7.60 (d, J = 15.7)/δ 7.13 (d, J = 2.2)/δ 7.06 (dd, J = 8.2, 2.2)/δ 6.86 (d, J = 8.3)/δ 6.33 (d, J = 15.9)/δ 5.30 (td, J = 4.9, 10.9)/δ 4.18 (br q, J = 3.1) |
| Phenylethanoid glucosides | ||||
| Verbascoside | ++ | ++ | +++ | δ 7.63 (d, J = 15.9)/δ 7.14 (d, J = 2.0)/7.05 (dd, J = 8.3, 2.0)/δ 6.67 (dd, J = 8.3, 2.0)/δ 6.34 (d, J = 15.9)/4.93 (t, J = 9.6)/4.47 (d, J = 7.9)/δ 2.81 (t, J = 7.2) 1.04 (d, J = 6.4) |
| Leucosepthoside A | + | + | + | δ 7.70 (d, J = 15.8)/δ 7.23 (d, J = 1.9)/7.16 (dd, J = 8.3, 2.0)/δ 6.89 (dd, J = 8.3, 2.0)/δ 6.41 (d, J = 16.0)/4.93 (t, J = 9.6)/4.47 (d, J = 7.9)/δ 3.88 (s)/ δ 2.81 (t, J = 7.1) 1.04 (d, J = 6.4) |
| Alkaloids | ||||
| Trigonelline | + | + | + | δ 9.12 (s)/δ 8.83 (m)/δ 8.07 (m)/δ 4.43 (s) |
| Others | ||||
| Choline | + | + | + | δ 3.19 (s) |
| Unidentified phenolic compounds | - | - | ++ | δ 7.99 (d, J = 8.9)/δ 7.10 (d, J = 8.9) δ 7.93 (d, J = 8.9)/δ 6.99 (d, J = 8.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovska, D.I.; Zhiponova, M.K.; Georgiev, M.I.; Alipieva, K.; Tsacheva, I.; Simova, S.; Yordanova, Z.P. Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov. Metabolites 2022, 12, 251. https://doi.org/10.3390/metabo12030251
Mantovska DI, Zhiponova MK, Georgiev MI, Alipieva K, Tsacheva I, Simova S, Yordanova ZP. Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov. Metabolites. 2022; 12(3):251. https://doi.org/10.3390/metabo12030251
Chicago/Turabian StyleMantovska, Desislava I., Miroslava K. Zhiponova, Milen I. Georgiev, Kalina Alipieva, Ivanka Tsacheva, Svetlana Simova, and Zhenya P. Yordanova. 2022. "Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov" Metabolites 12, no. 3: 251. https://doi.org/10.3390/metabo12030251
APA StyleMantovska, D. I., Zhiponova, M. K., Georgiev, M. I., Alipieva, K., Tsacheva, I., Simova, S., & Yordanova, Z. P. (2022). Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov. Metabolites, 12(3), 251. https://doi.org/10.3390/metabo12030251