Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid
Abstract
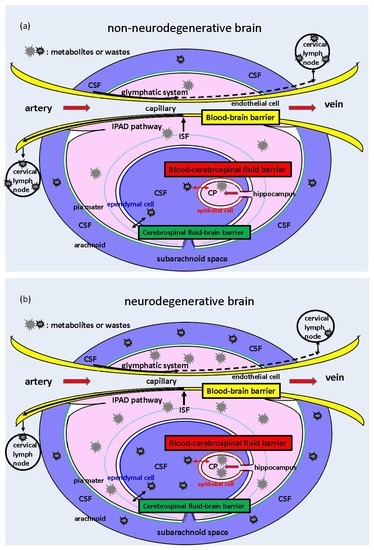
:1. Introduction
2. Glucose- and Neurotransmitter-Related CSF Metabolites
2.1. Glucose-Related CSF Metabolites
2.1.1. Glucose
2.1.2. Fructose
2.1.3. Urate
2.1.4. Lactate
2.2. Dopaminergic and Serotonergic Neurotransmitter-Related CSF Metabolites
3. Changes in CSF Compounds Caused by Neurodegeneration
3.1. Alzheimer’s Disease-Related Substances
3.2. Parkinson’s Disease and Other Neurodegenerative-Disease-Related Substances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ1-42 | amyloid-β(1-42) |
| ABCG2 | ATP-binding cassette transporter G2 |
| AD | Alzheimer’s disease |
| AE2 | anion exchange protein 2 |
| APP | amyloid precursor protein |
| AQP1 | aquaporin 1 |
| BBB | blood–brain barrier |
| BCRP | breast cancer resistance protein |
| BCSFB | blood–cerebrospinal fluid barrier |
| CBD | corticobasal degeneration |
| CBS | corticobasal syndrome |
| Ccf-mtDNA | circulating cell-free mitochondrial DNA |
| CP | choroid plexus |
| CPE | choroid plexus epithelial |
| CSF | cerebrospinal fluid |
| DLB | dementia with Lewy bodies |
| FDG | fluorodeoxyglucose |
| FTD | frontotemporal dementia |
| GLUT | glucose transporter |
| HIAA | hydroxyindoleacetic acid |
| iNPH | idiopathic normal pressure hydrocephalus |
| IPAD | intravascular periarterial drainage |
| ISF | interstitial fluid |
| miRNA | microRNA |
| MS | multiple sclerosis |
| MSA | multiple system atrophy |
| NfL | neurofilament light chain |
| Nlgn1 | neuroligin-1 |
| NMR | nuclear magnetic resonance |
| PD | Parkinson’s disease |
| PSP | progressive supranuclear palsy |
| P-tau | Phosphorylated tau |
| SGLT2 | sodium/glucose cotransporter 2 |
| S100B | calcium-binding protein B |
| T-tau | total tau |
| VDBT | vascular dementia of the Binswanger’s type |
References
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Brito, M.A.; Pahnke, J.; Santos, C.; Correia-Neves, M.; Palha, J.A. The choroid plexus in health and in disease: Dialogues into and out of the brain. Neurobiol. Dis. 2017, 107, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 1–33. [Google Scholar]
- Chiba, Y.; Murakami, R.; Matsumoto, K.; Wakamatsu, K.; Nonaka, W.; Uemura, N.; Yanase, K.; Kamada, M.; Ueno, M. Glucose, fructose, and urate transporters in the choroid plexus epithelium. Int. J. Mol. Sci. 2020, 21, 7230. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davson, H.; Welch, K.; Segal, M.B. Chemical composition and secretory nature of the fluid. In The Physiology and Pathophysiology of the Cerebrospinal Fluid; Davson, H., Welch, K., Segal, M.B., Eds.; Churchill Livingstone: Edinburgh, Scotland; London, UK; Melbourne, Australia; New York, NY, USA, 1987; pp. 15–63. [Google Scholar]
- Liao, C.-C.; Hou, T.-H.; Yu, H.-P.; Li, A.; Liu, F.-C. Cerebrospinal fluid electrolytes and acid-base in diabetic patients. Transl. Neurosci. 2021, 12, 448–455. [Google Scholar] [CrossRef]
- Cserr, H.F.; Knopf, P.M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: A new view. Immunol. Today 1992, 13, 507–512. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Jeffrey, M.; Kalaria, R.N.; Weller, R.O. Cerebral amyloid angiopathy, prion angiopathy, CADASIL and spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 2013, 39, 593–611. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Weller, R.O. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav. Immun. 2014, 36, 9–14. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—Implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliff, J.J.; Goldman, S.A.; Nedergaard, M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015, 14, 977–979. [Google Scholar] [CrossRef] [Green Version]
- Lach, B.; Sceithauer, B.W.; Gregor, A.; Wick, M.R. Colloid cyst of the third ventricle. A comparative immunohistochemical study of neuraxis cysts and choroid plexus epithelium. J. Neurosurg. 1993, 78, 101–111. [Google Scholar] [CrossRef]
- Shibahara, J.; Kashima, T.; Kikuchi, Y.; Kunita, A.; Fukayama, M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Archiv. 2006, 448, 493–499. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Miyai, Y.; Kawauchi, M.; Yanase, K.; Uemura, N.; Ueno, M. Immunohistochemical expression of osteopontin and collagens in choroid plexus of human brains. Neuropathology 2021. [Google Scholar] [CrossRef]
- Christensen, I.B.; Mogensen, E.N.; Damkier, H.H.; Praetorius, J. Choroid plexus epithelial cells express the adhesion protein P-cadherin at cell-cell contacts and syntazin-4 in the luminal membrane domain. Am. J. Physiol. Cell Physiol. 2018, 314, C519–C533. [Google Scholar] [CrossRef]
- Christensen, I.B.; Gyldenholm, T.; Damkier, H.H.; Praetorius, J. Polarization of membrane associated proteins in the choroid plexus epithelium from normal and slc4a10 knockout mice. Front. Physiol. 2013, 4, 344. [Google Scholar] [CrossRef] [Green Version]
- Lippoldt, A.; Liebner, S.; Andbjer, B.; Kalbacher, H.; Wolburg, H.; Haller, H.; Fuxe, K. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2, and -5 expression by protein kinase C. Neuroreport 2000, 11, 1427–1431. [Google Scholar] [CrossRef]
- Steinemann, A.; Galm, I.; Chip, S.; Nitsch, C.; Maly, I.P. Claudin-1, -2, -3 are selectively expressed in the epithelia of the choroid plexus of the mouse from early development and into adulthood while Claudin-5 is restricted to endothelial cells. Front. Neuroanat. 2016, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schlzke, J.D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; A comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 1–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology 2020, 11, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 1–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Cauwenberghe, C.; Gorle, N.; Vandenbroucke, R.E. Role of the Choroid Plexus in Aging. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 209–232. [Google Scholar]
- Balusu, S.; Brkic, M.; Libert, C.; Vandenbroucke, R.E. The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: More than just a barrier. Neural Regen. Res. 2016, 11, 534–537. [Google Scholar] [PubMed]
- Leen, W.G.; Willemsen, M.A.; Wevers, R.A.; Verbeek, M.M. Cerebrospinal fluid glucose and lactate: Age-specific reference values and implications for clinical practice. PLoS ONE 2012, 7, e42745. [Google Scholar] [CrossRef] [Green Version]
- Thanh, T.T.; Casals-Pascual, C.; Ny, N.T.H.; Ngoc, N.M.; Geskus, R.; Nhu, L.N.T.; Hong, N.T.T.; Duc, D.T.; Thu, D.D.A.; Uyen, P.N.; et al. Value of lipocalin 2 as a potential biomarker for bacterial meningitis. Clin. Microbiol. Infect. 2021, 27, 724–730. [Google Scholar] [CrossRef]
- Daouk, J.; Bouzarar, R.; Chaarani, B.; Zmudka, J.; Meyer, M.E.; Baledent, O. Use of dynamic (18)F-fluorodeoxyglucose positron emission tomography to investigate choroid plexus function in Alzheimer’s disease. Exp. Gerontol. 2016, 77, 62–68. [Google Scholar] [CrossRef]
- Pappas, C.; Klinedinst, B.S.; Le, S.; Wang, Q.; Larsen, B.; McLimans, K.; Lockhart, S.N.; Allenspach-Jorn, K.; Mochel, J.P.; for the Alzheimer’s Disease Neuroimaging Initiative. CSF glucose tracks regional tau progression based on Alzheimer’s disease risk factors. Alzheimers Dement. 2020, 6, e12080. [Google Scholar] [CrossRef]
- Öhman, A.; Forsgren, L. NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson’s disease and controls. Neurosci. Lett. 2015, 594, 36–39. [Google Scholar] [CrossRef]
- Kawasaki, T.; Akanuma, H.; Yamanouchi, T. Increased fructose concentrations in blood and urine in patients with diseases. Diabetes Care 2002, 25, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [Green Version]
- Lutz, N.W.; Viola, A.; Malikova, I.; Confort-Gouny, S.; Audoin, B.; Ranjeva, J.P.; Pelletier, J.; Cozzone, P.J. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLoS ONE 2007, 2, e595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.J.; Johnson, A.; Cline, G.; Belfort-DeAguiar, R.; Snegovskikh, D.; Khokhar, B.; Han, C.S.; Sherwin, R.S. Fructose levels are markedly elevated in cerebrospinal fluid compared to plasma in pregnant women. PLoS ONE 2015, 10, e0128582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Slone, J.; Song, X.; Amrein, H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 2012, 151, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Nishi, N.; Nakagawa, T.; Chiba, Y.; Tsukamoto, I.; Kusaka, T.; Miki, T.; Sakamoto, H.; Yamaguchi, F.; Tokuda, M. Immunoreactivity of glucose transporter 5 is located in epithelial cells of the choroid plexus and ependymal cells. Neuroscience 2014, 260, 149–157. [Google Scholar] [CrossRef]
- Murakami, R.; Chiba, Y.; Tsuboi, K.; Matsumoto, K.; Kawauchi, M.; Fujihara, R.; Mashima, M.; Kanenishi, K.; Yamamoto, T.; Ueno, M. Immunoreactivity of glucose transporter 8 is localized in epithelial cells of the choroid plexus and in ependymal cells. Histochem. Cell Biol. 2016, 146, 231–236. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Dziegielewska, K.M.; Ek, C.J.; Habgood, M.D.; Bauer, H.; Bauer, H.C.; Lindsay, H.; Wakefield, M.J.; Strazielle, N.; Kratzer, I.; et al. Mechanisms that determine the internal environment of the developing brain: A transcriptomic, functional and ultrastructural approach. PLoS ONE 2013, 8, e65629. [Google Scholar] [CrossRef] [Green Version]
- Oppelt, S.A.; Zhang, W.; Tolan, D.R. Specific regions of the brain are capable of fructose metabolism. Brain Res. 2017, 1657, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Trezzi, J.-P.; Galozzi, S.; Jaeger, C.; Barkovits, K.; Brockmann, K.; Maetzler, W.; Berg, D.; Marcus, K.; Betsou, F.; Hiller, K.; et al. Distinct metabolomic signature in cerebrospinal fluid in early parkinson’s disease. Mov. Disord. 2018, 32, 1401–1408. [Google Scholar] [CrossRef]
- Regenold, W.T.; Phatak, P.; Makley, M.J.; Stone, R.D.; Kling, M.A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 2008, 275, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Oppendisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric acid as a CNS antioxidant. J. Alzheimers Dis. 2010, 19, 1331–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.; Li, H.; Zhang, L.; Wang, Y.; Long, Y.; Li, R.; Qiu, W.; Lu, Z.; Hu, X.; Peng, F. Elevated cerebrospinal fluid uric acid during relapse of neuromyelitis optica spectrum disorders. Brain Behav. 2017, 7, e00584. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.A.; Phillis, J.W.; O’Reagan, M.H. Determination of rat cerebrospinal fluid concentrations of adenosine, inosine, hypoxanthine, xanthine and uric acid by high performance liquid chromatography. J. Pharm. Pharmacol. 1988, 40, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Lu, W.; Gao, F.; Li, D.; Hu, J.; Li, Y.; Zuo, Z.; Jie, H.; Zhao, Y.; Cen, X. Uric acid induces cognitive dysfunction through hippocampal inflammation in rodents and humans. J. Neurosci. 2016, 36, 10990–11005. [Google Scholar] [CrossRef] [Green Version]
- Uemura, N.; Murakami, R.; Chiba, Y.; Yanase, K.; Fujihara, R.; Mashima, M.; Matsumoto, K.; Kawauchi, M.; Shirakami, G.; Ueno, M. Immunoreactivity of urate transporters, GLUT9 and URAT1, is located in epithelial cells of the choroid plexus of human brains. Neurosci. Lett. 2017, 659, 99–103. [Google Scholar] [CrossRef]
- Tomioka, N.H.; Tamura, Y.; Takada, T.; Shibata, S.; Suzuki, H.; Uchida, S.; Hosoyamada, M. Immunohistochemical and in situ hybridization study of urate transporters GLUT9/URATv1, ABCG2, and URAT1 in the murine brain. Fluids Barriers CNS 2016, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, localization, and species differences at the blood-brain and the blood-Cerebrospinal Fluid Barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef]
- Tomioka, N.H.; Nakamura, M.; Doshi, M.; Deguchi, Y.; Ichida, K.; Morisaki, T.; Hosoyamada, M. Ependymal cells of the mouse brain express urate transporter 1 (URAT1). Fluids Barriers CNS 2013, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Simon, K.C.; Eberly, S.; Gao, X.; Oakes, D.; Tanner, C.M.; Shoulson, I.; Fahn, S.; Schwarzschild, M.A.; Ascherio, A.; Parkinson Study Group. Mendelian randomization of serum urate and Parkinson disease progression. Ann. Neurol. 2014, 76, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.Y.; Lan, T.Y.; Tang, G.J.; Tang, C.H.; Chen, T.J.; Lin, H.Y. Gout and the risk of dementia: A nationwide-based cohort study. Arthritis Res. Ther. 2015, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohgi, H.; Abe, T.; Takahashi, S.; Kikuchi, T. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J. Neural. Transm. Park. Dis. Dement. Select. 1993, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shahan, B.; Choi, E.Y.; Nieves, G. Cerebrospinal fluid analysis. Am. Fam. Physician 2021, 103, 422–428. [Google Scholar] [PubMed]
- Murakami, R.; Chiba, Y.; Nishi, N.; Matsumoto, K.; Wakamatsu, K.; Yanase, K.; Uemura, N.; Nonaka, W.; Ueno, M. Immunoreactivity of receptor and transporters for lactate located in astrocytes and epithelial cells of choroid plexus of human brain. Neurosci. Lett. 2021, 741, 135479. [Google Scholar] [CrossRef]
- Lin, H.-T.; Cheng, M.-L.; Lo, C.-J.; Hsu, W.-C.; Lin, G.; Liu, F.-C. 1H NMR metabolomic profiling of human cerebrospinal fluid in aging process. Am. J. Transl. Res. 2021, 13, 12495–12508. [Google Scholar]
- Liguori, C.; Stefani, A.; Fernandes, M.; Cerroni, R.; Merculi, N.B.; Pierantozzi, M. Biomarkers of cerebral glucose metabolism and neurodegeneration in Parkinson’s disease: A cerebrospinal fluid-based study. J. Parkinsons Dis. 2022, 12, 537–544. [Google Scholar] [CrossRef]
- Bonomi, C.G.; Lucia, V.D.; Mascolo, A.P.; Assogna, M.; Motta, C.; Scaricamazza, E.; Sallustio, F.; Mercuri, N.B.; Koch, G.; Martorana, A. Brain energy metabolism and neurodegeneration: Hints from CSF lactate levels in dementias. Neurobiol. Aging 2021, 105, 333–339. [Google Scholar] [CrossRef]
- Andersen, A.D.; Binzer, M.; Stenager, E.; Gramsbergen, J.B. Cerebrospinal fluid biomarkers for Parkinson’s disease—A systematic review. Acta Neurol. Scand. 2017, 135, 34–56. [Google Scholar] [CrossRef]
- Kaiserova, M.; Chudackova, M.; Vranova, H.P.; Mensikova, K.; Vastik, M.; Kastelikova, A.; Stejskal, D.; Kanovsky, P. Cerebrospinal fluid levels of 5-Hydroxyindoleacetic acid in Parkinson’s disease and atypical Parkinsonian syndromes. Neurodegener. Dis. 2021, 21, 30–35. [Google Scholar] [CrossRef]
- Serot, J.M.; Bene, M.C.; Faure, G.C. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front. Biosci. 2003, 8, s515–s521. [Google Scholar] [PubMed] [Green Version]
- Gonzalez-Marrero, I.; Gimenez-Llort, L.; Johanson, C.E.; Carmona-Calero, E.M.; Castaneyra-Ruiz, L.; Brito-Armas, J.M.; Cataneyra-Perdomo, A.; Castro-Fuentes, R. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Bridel, C.; Somers, C.; Sieben, A.; Rozemuller, A.; Niemantsverdriet, E.; Stuyfs, H.; Vermeiren, Y.; van Vermeiren, Y.; van Broeckhoven, C.; De Deyn, P.P.; et al. Associating Alzheimer’s disease pathology with its cerebrospinal fluid biomarkers. Brain 2022, awac013. [Google Scholar] [CrossRef] [PubMed]
- Angello, L.; Gambino, C.M.; Lo Sasso, B.; Bivona, B.; Milano, S.; Ciaccio, A.M.; Piccolo, T.; La Bella, V.; Ciaccio, M. Neurogranin as a novel biomarker in Alzheimer’s disease. Lab. Med. 2021, 52, 188–196. [Google Scholar]
- Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Piccoli, T.; Gambino, C.M.; Bivona, G.; Giglio, R.V.; Ciaccio, A.M.; La Bella, V.; et al. Neurogranin as a reliable biomarker for synaptic dysfunction in Alzheimer’s disease. Diagnistics 2021, 11, 2339. [Google Scholar] [CrossRef]
- Yoong, S.Q.; Lu, J.; Xing, H.; Gyanwali, B.; Tan, Y.Q.; Wu, X.V. The prognostic utility of CSF neurogranin in predicting future cognitive decline in the Alzheimer’s disease continuum: A systematic review and meta-analysis with narrative synthesis. Ageing Res. Rev. 2021, 72, 101491. [Google Scholar] [CrossRef]
- Camporesi, E.; Lashley, T.; Gobom, J.; Lantero-Rodriguez, J.; Hansson, O.; Zetterberg, H.; Blennow, K.; Becker, B. Neuroligin-1 in brain and CSF of neurodegenerative disorders: Investigation for synaptic biomarkers. Acta Neuropathol. Commun. 2021, 9, 19. [Google Scholar] [CrossRef]
- Kawamura, K.; Miyajima, M.; Nakajima, M.; Kanai, M.; Motoi, Y.; Nojiri, S.; Akiba, C.; Ogino, I.; Xu, H.; Kamohara, C.; et al. Cerebrospinal fluid amyloid-β oligomer levels in patients with idiopathic normal pressure hydrocephalus. J. Alzheimers Dis. 2021, 83, 179–190. [Google Scholar] [CrossRef]
- Lee, C.Y.; Ryu, I.S.; Ryu, J.-H.; Cho, H.-J. mi RNAs as therapeutic tools in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 13012. [Google Scholar] [CrossRef]
- Tan, Y.J.; Wong, B.Y.X.; Vaidyanathan, R.; Sreejith, S.; Chia, S.Y.; Kandiah, N.; Ng, A.S.L.; Zeng, L. Altered cerebrospinal fluid exosomal microRNA levels in young-onset Alzheimer’s disease and frontotemporal dementia. J. Alzheimers Dis. Rep. 2021, 5, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, K.; Ito, H.; Abe, E.; Fuwa, T.J.; Kanno, M.; Murakami, Y.; Abe, M.; Murakami, T.; Yoshihara, A.; Ugawa, Y.; et al. Transferrin biosynthesized in the brain is a novel biomarker for Alzheimer’s disease. Metabolites 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Podlesniy, P.; Llorens, F.; Puigròs, M.; Serra, N.; Sepúlveda-Falla, D.; Schmidt, C.; Hermann, P.; Zerr, I.; Trullas, R. Cerebrospinal fluid mitochondrial DNA in rapid and slow progreesive forms of Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 6298. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Doring, F.; Trenkwalder, C.; Schlossmacher, M.G. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef]
- Shi, M.; Bradner, J.; Hancock, A.M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Kim, H.M.; et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 2011, 69, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Constantinides, V.C.; Majbour, N.K.; Paraskevas, G.P.; Abdi, I.; Safieh-Garabedian, B.; Stefanis, L.; El-Agnaf, O.M.; Kapaki, E. Cerebrospinal fluid α-synuclein species in cognitive and movements disorders. Brain Sci. 2021, 11, 119. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic assessment of 10 biomarker candidates focusing on α-synuclein-related disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Kazantsev, A.G.; Kolchinsky, A.M. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Archiv. Neurol. 2008, 65, 1577–1581. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Zetterberg, H.; Brix, B.; Mattsson, N.; Surova, Y.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Movement Dis. 2020, 35, 513–518. [Google Scholar] [CrossRef]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlated with disease status and features of pathology. PLoS ONE 2014, 9, e94839. [Google Scholar]
- Lowes, H.; Pyle, A.; Santibanez-Koref, M.; Hudson, G. Circulating cell-free mitochondrial DNA levels in Parkinson’s disease are influenced by treatment. Mol. Neurodegener. 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaby, C.; Alcolea, D.; Carmona-Iragui, M.; Illán-Gala, I.; Morenas-Rodríguez, E.; Barroeta, I.; Altuna, M.; Estellés, T.; Santos-Santos, M.; Turon-Sans, J.; et al. Differential levels of neurofilament light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Sci. Rep. 2020, 10, 9161. [Google Scholar] [CrossRef] [PubMed]
- Kaiserova, M.; Chudackova, M.; Mensikova, K.; Vastik, M.; Kurcova, S.; Vranova, H.P.; Stejskal, D.; Kanovsky, P. Cerebrospinal fluid levels of chromogranin A in Parkinson’s disease and multiple system atrophy. Brain Sci. 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; Bang, J.; Lobach, I.V.; Tsai, R.M.; Rabinovici, G.D.; Miller, B.L.; Boxer, A.L.; on behalf of AL-108-231 Investigators. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018, 90, e273–e281. [Google Scholar] [CrossRef]
- Nonaka, W.; Takata, T.; Iwama, H.; Komatsubara, S.; Kobara, H.; Kamada, M.; Deguchi, K.; Touge, T.; Miyamoto, O.; Nakamura, T.; et al. A cerebrospinal fluid microRNA analysis: Progressive supranuclear palsy. Mol. Med. Rep. 2022, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, A.; Zambrano, K.; Sanon, S.; Gavilanes, A.W.D. Extracellular mitochondria in the cerebrospinal fluid (CSF): Potential types and key roles in central nervous system (CNS) physiology and pathogenesis. Mitochondrion 2021, 58, 255–269. [Google Scholar] [CrossRef]


| Molecules | Apical Side | Basal Side |
|---|---|---|
| H2O | AQP1 | AQP1 |
| Na+, K+ | Na+−K+−ATPase | |
| Na+, K+, 2Cl− | NKCC1 | |
| Na+, H+ | NHE1 | |
| Na+, HCO3− | NBCe2 | NBCn1 |
| Na+, Cl−, HCO3− | Ncbe | |
| Cl−, HCO3− | AE2 | |
| Cl− | Clir, VRAC | |
| K+ | Kir7.1, Kv |
| Ions and Osm. | Rabbit 4,7 | Dog 7 | Human 7 | Human 8 |
|---|---|---|---|---|
| Na+, mEq/L | 149 | 151 | 147 | 137 ± 1.8 |
| K+, mEq/L | 2.9 | 2.98 | 2.9 | 2.8 ± 0.1 |
| Cl−, mEq/L | 130 | 132.5 | 113 | 122 ± 1.9 |
| pH | 7.27 | 7.42 | 7.31 | 7.43 ± 0.02 |
| Osm. (mOsm/L) | 305.2 | 305.2 | 289 | n/a |
| CSF Compounds | Related Disorders | Expression | Cited Papers | |
|---|---|---|---|---|
| (a-1) | glucose | aging | inc | [29] |
| diabetes mellitus | inc | [30] | ||
| bacterial meningitis | dec | [31] | ||
| Parkinson’s disease | dec | [33] | ||
| (a-2) | fructose | Parkinson’s disease | inc | [43] |
| multiple sclerosis | inc | [44] | ||
| (a-3) | urate | BBB impairment | inc | [46] |
| VDBT | inc | [56] | ||
| (a-4) | lactate | aging | inc | [29,59] |
| Parkinson’s disease | inc | [60] | ||
| multiple sclerosis | inc | [44] | ||
| (b-1) | dopaminergic | Parkinson’s disease | dec | [62] |
| metabolites | ||||
| (b-2) | 5-HIAA | Parkinson’s disease | dec | [63] |
| MSA | dec | [63] |
| Diseases | Related CSF Compounds | inc/dec | Cited Papers | |
|---|---|---|---|---|
| (a) | Alzheimer’s | amyloid-b (1-42) | dec | [66,67] |
| disease | total tau | inc | [66,67] | |
| phosphorylated tau | inc | [66,67] | ||
| neurogranin | inc | [68,69] | ||
| neuroligin-1 | dec | [71] | ||
| microRNAs | inc/dec | [73,74] | ||
| Man-transferrin | inc | [75] | ||
| ccf-mtDNA | dec | [76] | ||
| (b) | Parkinson’s | total α-synuclein | dec | [77,78,79,80] |
| disease | p-α-synuclein | inc | [79,81] | |
| oligomeric a-synuclein | inc | [62,82] | ||
| NfL | n.s./inc | [62,80] | ||
| S100B | dec | [80] | ||
| neurogranin | dec | [83] | ||
| microRNAs | inc/dec | [84] | ||
| ccf-mtDNA | dec | [85] | ||
| (c) | Parkinsonism | α-synuclein | dec | [77,78,79,80] |
| NfL | inc | [80,86] | ||
| neurogranin | dec | [83] | ||
| (DLB) | S100B | inc | [80] | |
| (MSA) | chromogranin A | dec | [87] | |
| (PSP) | NfL/p-tau ratio | inc | [88] | |
| microRNAs | inc/dec | [89] | ||
| (iNPH) | Ab oligomers (10-20) | inc | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, K.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Kamada, M.; Nonaka, W.; Uemura, N.; Yanase, K.; Ueno, M. Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid. Metabolites 2022, 12, 343. https://doi.org/10.3390/metabo12040343
Wakamatsu K, Chiba Y, Murakami R, Miyai Y, Matsumoto K, Kamada M, Nonaka W, Uemura N, Yanase K, Ueno M. Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid. Metabolites. 2022; 12(4):343. https://doi.org/10.3390/metabo12040343
Chicago/Turabian StyleWakamatsu, Keiji, Yoichi Chiba, Ryuta Murakami, Yumi Miyai, Koichi Matsumoto, Masaki Kamada, Wakako Nonaka, Naoya Uemura, Ken Yanase, and Masaki Ueno. 2022. "Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid" Metabolites 12, no. 4: 343. https://doi.org/10.3390/metabo12040343
APA StyleWakamatsu, K., Chiba, Y., Murakami, R., Miyai, Y., Matsumoto, K., Kamada, M., Nonaka, W., Uemura, N., Yanase, K., & Ueno, M. (2022). Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid. Metabolites, 12(4), 343. https://doi.org/10.3390/metabo12040343