Identification of Metabolomic Biomarkers of Long-Term Stress Using NMR Spectroscopy in a Diving Duck
Abstract
:1. Introduction
2. Results
2.1. Serum CORT
2.2. Serum Metabolites
2.3. Body Weight
3. Discussion
4. Materials and Methods
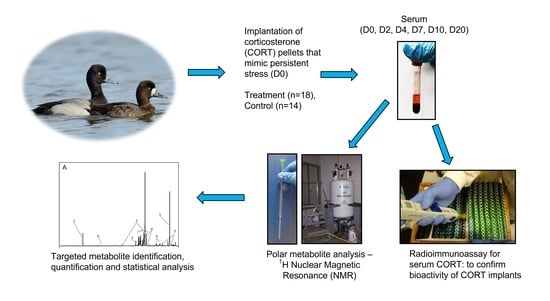
4.1. Study Area and Fieldwork
4.2. Experimental Design
4.3. Surgical Procedure for Corticosterone Pellet Implantation
4.4. Sample Collection and Storage
4.5. Radioimmunoassay for Serum Corticosterone
4.6. Sample Extraction and Preparation for NMR
4.7. Data Processing and Statistical Analysis
4.7.1. Effect of CORT Treatment on Serum CORT
4.7.2. Serum Metabolites
4.7.3. Body Weight
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sih, A.; Ferrari, M.C.O.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, J.C.; Maney, D.L.; Breuner, C.W.; Jacobs, J.D.; Lynn, S.; Ramenofsky, M.; Richardson, R.D. Ecological Bases of Hormone-Behavior Interactions: The “Emergency Life History Stage”. Am. Zool. 1998, 38, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Fairhurst, G.D.; Marchant, T.A.; Soos, C.; Machin, K.L.; Clark, R.G. Experimental relationships between levels of corticosterone in plasma and feathers in a free-living bird. J. Exp. Biol. 2013, 216, 4071–4081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thierry, A.-M.; Ropert-Coudert, Y.; Raclot, T. Elevated corticosterone levels decrease reproductive output of chick-rearing Adélie penguins but do not affect chick mass at fledging. Conserv. Physiol. 2013, 1, 10. [Google Scholar] [CrossRef]
- Almasi, B.; Roulin, A.; Jenni, L. Corticosterone shifts reproductive behaviour towards self-maintenance in the barn owl and is linked to melanin-based coloration in females. Horm. Behav. 2013, 64, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Kitaysky, A.S.; Kitaiskaia, E.V.; Piatt, J.F.; Wingfield, J.C. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 2003, 43, 140–149. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Reina, R.D.; Lill, A. Interpreting indices of physiological stress in free-living vertebrates. J. Comp. Physiol. B 2012, 182, 861–879. [Google Scholar] [CrossRef]
- Henry, J.P. Biological basis of the stress response. Integr. Physiol. Behav. Sci. 1992, 27, 66–83. [Google Scholar] [CrossRef]
- Novais, A.; Monteiro, S.; Roque, S.; Correia-Neves, M.; Sousa, N. How age, sex and genotype shape the stress response. Neurobiol. Stress 2017, 6, 44–56. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gormally, B.M.G.; Romero, L.M. What are you actually measuring? A review of techniques that integrate the stress response on distinct time-scales. Funct. Ecol. 2020, 34, 2030–2044. [Google Scholar] [CrossRef]
- Bundy, J.; Davey, M.; Viant, M. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3–21. [Google Scholar] [CrossRef]
- Samuelsson, L.M.; Larsson, D.G.J. Contributions from metabolomics to fish research. Mol. BioSyst. 2008, 4, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Ubach, A.; Pérez-Trujillo, M.; Sardans, J.; Gargallo-Garriga, A.; Parella, T.; Peñuelas, J. Ecometabolomics: Optimized NMR-based method. Methods Ecol. Evol. 2013, 4, 464–473. [Google Scholar] [CrossRef]
- Miller, M.G. Environmental Metabolomics: A SWOT Analysis (Strengths, Weaknesses, Opportunities, and Threats). J. Proteome Res. 2007, 6, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R. Recent developments in environmental metabolomics. Mol. BioSyst. 2008, 4, 980–986. [Google Scholar] [CrossRef]
- Pomfret, S.M.; Brua, R.B.; Izral, N.M.; Yates, A.G. Metabolomics for biomonitoring: An evaluation of the metabolome as an indicator of aquatic ecosystem health. Environ. Rev. 2020, 28, 89–98. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Mappley, L.J.; La Ragione, R.M.; Woodward, M.J.; Claus, S.P. NMR-based metabolic characterization of chicken tissues and biofluids: A model for avian research. Metabolomics 2016, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Beauclercq, S.; Nadal-Desbarats, L.; Hennequet-Antier, C.; Gabriel, I.; Tesseraud, S.; Calenge, F.; Le Bihan-Duval, E.; Mignon-Grasteau, S. Relationships between digestive efficiency and metabolomic profiles of serum and intestinal contents in chickens. Sci. Rep. 2018, 8, 6678. [Google Scholar] [CrossRef] [Green Version]
- Metzler-Zebeli, B.U.; Siegerstetter, S.C.; Magowan, E.; Lawlor, P.G.; O’Connell, N.E.; Zebeli, Q. Feed Restriction Reveals Distinct Serum Metabolome Profiles in Chickens Divergent in Feed Efficiency Traits. Metabolites 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.C.; Luo, Y.T.; Wang, G.Y.; Ge, C.R.; Zhou, G.H.; Zhang, W.G.; Liao, G.Z. H-1-NMR-based water-soluble low molecular weight compound characterization and fatty acid composition of boiled Wuding chicken during processing. J. Sci. Food Agric. 2019, 99, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadde, U.D.; Oh, S.; Lillehoj, H.S.; Lillehoj, E.P. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci. Rep. 2018, 8, 3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Serum metabolomics study of nutrient metabolic variations in chronic heat-stressed broilers. Br. J. Nutr. 2018, 119, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaytsoff, S.J.M.; Brown, C.L.J.; Montina, T.; Metz, G.A.S.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef]
- Anteau, M.J.; DeVink, J.; Koons, D.N.; Austin, J.E.; Custer, C.M.; Afton, A.D. Lesser Scaup (Aythya affinis). In Birds of the World, Version 1.0; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Greenberg, N.; Carr, J.A.; Summers, C.H. Causes and Consequences of Stress. Integr. Comp. Biol. 2002, 42, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Blas, J. Chapter 33—Stress in Birds. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, MA, USA, 2015; pp. 769–810. [Google Scholar]
- Langslow, D.R. Gluconeogenesis in Birds. Biochem. Soc. Trans. 1978, 6, 1148–1152. [Google Scholar] [CrossRef] [Green Version]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, W.H. On the biosynthesis of purines in the bird; role of formate. J. Biol. Chem. 1951, 190, 633–641. [Google Scholar] [CrossRef]
- Oizel, K.; Tait-Mulder, J.; Fernandez-de-Cossio-Diaz, J.; Pietzke, M.; Brunton, H.; Lilla, S.; Dhayade, S.; Athineos, D.; Blanco, G.R.; Sumpton, D.; et al. Formate induces a metabolic switch in nucleotide and energy metabolism. Cell Death Dis. 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-S.; Xu, H.-B.; Huang, K.-X.; Hu, W.-H.; Liu, M.-L. A study on human urine in a high-selenium area of china by 1H-NMR spectroscopy. Biol. Trace Elem. Res. 2002, 89, 155–163. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, X.; Ma, C.; Zhao, J.; Xiong, Z. 1H-NMR-based urinary metabolomic analysis for the preventive effects of gushudan on glucocorticoid-induced osteoporosis rats. Anal. Biochem. 2020, 610, 113992. [Google Scholar] [CrossRef]
- Beers, K.W.; Raup, T.J.; Bottje, W.G.; Odom, T.W. Physiological responses of heat-stressed broilers fed nicarbazin. Poult. Sci. 1989, 68, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Y.; Sheng, Z.; Liu, G.; Fu, Z.; Zhao, J.; Zhao, J.; Yan, X.; Zhu, B.; Peng, S. NMR-based metabonomic analysis of the hepatotoxicity induced by combined exposure to PCBs and TCDD in rats. Toxicol. Appl. Pharmacol. 2010, 248, 178–184. [Google Scholar] [CrossRef]
- Gladden, L. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Yanxiang Guo, J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Pollock, C. Carbohydrate regulation in avian species. J. Exot. Pet Med. 2002, 11, 57–64. [Google Scholar] [CrossRef]
- Kidd, M.T.; Kerr, B.J. L-Threonine for Poultry: A Review. J. Appl. Poult. Res. 1996, 5, 358–367. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, T.; Qiu, Y.; Su, M.; Jiang, T.; Zhou, M.; Zhao, A.; Jia, W. Metabonomics Approach to Understanding Acute and Chronic Stress in Rat Models. J. Proteome Res. 2009, 8, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Q.; Xiao, J.; Jiao, H.; Lin, H. Glucocorticoids retard skeletal muscle development and myoblast protein synthesis through a mechanistic target of rapamycin (mTOR)-signaling pathway in broilers (Gallus gallus domesticus). Int. J. Biol. Stress 2015, 18, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Klasing, K.C.; Laurin, D.E.; Peng, R.K.; Fry, D.M. Immunologically mediated growth depression in chicks: Influence of feed intake, corticosterone and interleukin-1. J. Nutr. 1987, 117, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Linder, S.J.; Mostoslavsky, R. Chapter 15—Interaction Between Cellular Metabolic States and Chromatin Dynamics. In Chromatin Regulation and Dynamics; Göndör, A., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 373–398. [Google Scholar]
- Gutiérrez, J.S.; Sabat, P.; Castañeda, L.E.; Contreras, C.; Navarrete, L.; Peña-Villalobos, I.; Navedo, J.G. Oxidative status and metabolic profile in a long-lived bird preparing for extreme endurance migration. Sci. Rep. 2019, 9, 17616. [Google Scholar] [CrossRef] [PubMed]
- Jenni-Eiermann, S.; Jenni, L. Metabolic responses to flight and fasting in night-migrating passerines. J. Comp. Physiol. B 1991, 161, 465–474. [Google Scholar] [CrossRef]
- Landys, M.M.; Piersma, T.; Guglielmo, C.G.; Jukema, J.; Ramenofsky, M.; Wingfield, J.C. Metabolic profile of long-distance migratory flight and stopover in a shorebird. Proc. Biol. Sci. 2005, 272, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Griminger, P. Lipid metabolism. In Avian Physiology; Sturkie, P.D., Ed.; Springer: New York, NY, USA, 1986; pp. 345–358. [Google Scholar]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef] [Green Version]
- Modica-Napolitano, J.S.; Renshaw, P.F. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: Implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol. Psychiatry 2004, 55, 273–277. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, M.; Lu, X.; Huo, T.; Ge, L.; Yang, J.; Wu, C.; Li, F. Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin. Chim. Acta 2010, 411, 204–209. [Google Scholar] [CrossRef]
- Dong, H.; Lin, H.; Jiao, H.C.; Song, Z.G.; Zhao, J.P.; Jiang, K.J. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Eid, Y.Z.; Ohtsuka, A.; Hayashi, K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br. Poult. Sci. 2003, 44, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sui, S.J.; Jiao, H.C.; Buyse, J.; Decuypere, E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 143, 400–405. [Google Scholar] [CrossRef] [PubMed]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “Stress” Are Not Synonymous. Integr. Org. Biol. 2019, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusani, L. Endocrinology in field studies: Problems and solutions for the experimental design. Gen. Comp. Endocrinol. 2008, 157, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Almasi, B.; Roulin, A.; Breuner, C.W.; Jenni-Eiermann, S.; Jenni, L. Effects of corticosterone pellets on baseline and stress-induced corticosterone and corticosteroid-binding-globulin. Gen. Comp. Endocrinol. 2009, 160, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, P.B.; Hollmén, T.E.; Atkinson, S.; Mashburn, K.L.; Tuomi, P.A.; Esler, D.; Mulcahy, D.M.; Rizzolo, D.J. Effects of ACTH, capture, and short term confinement on glucocorticoid concentrations in harlequin ducks (Histrionicus histrionicus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 275–283. [Google Scholar] [CrossRef]
- Viant, M.R. Environmental metabolomics using 1H-NMR spectroscopy. Methods Mol. Biol. 2008, 410, 137–150. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; van Ommen, B.; Smilde, A.K. Large-Scale Human Metabolomics Studies: A Strategy for Data (Pre-) Processing and Validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]





| No. | Compound Name | 1H NMR Chemical Shift (ppm) a, Multiplicity b |
|---|---|---|
| 1 | 2-Hydroxybutyrate | 0.88 t, 1.64 m, 1.73 m, 4.00 q |
| 2 | 3-Hydroxybutyrate | 1.19 d, 2.30 q, 2.40 q, 4.14 m |
| 3 | Acetate | 1.91 s |
| 4 | Acetoacetate | 2.27 s, 3.44 s |
| 5 | Alanine | 1.47 d, 3.78 q |
| 6 | Anserine | 2.65 m, 2.71 m, 3.05 q, 3.2 m, 3.78 s, 4.5q, 7.25 s, 7.92 s, 8.27 d |
| 7 | Arginine | 1.64 m, 1.70 m, 1.88 m, 1.92 m, 3.24 t, 3.76 t |
| 8 | Asparagine | 2.85 q, 2.94 q, 3.99 q, 6.91 s |
| 9 | Betaine | 3.26 s, 3.89 s |
| 10 | Carnitine | 2.41m, 3.21s, 3.42 m, 4.56 s |
| 11 | Choline | 3.19 s, 3.51 t, 4.06 s |
| 12 | Citrate | 2.53 d, 2.66 d |
| 13 | Creatine | 3.02 s, 3.92 s |
| 14 | Creatinine | 3.03 s, 4.05 s |
| 15 | Dimethylamine | 2.72 s |
| 16 | Ethanolamine | 3.13 t, 3.82 t |
| 17 | Formate | 8.44 s |
| 18 | Fumarate | 6.51 s |
| 19 | Glucose | 3.24 t, 3.40 m, 3.51 m, 3.70 m, 3.82 m, 3.89 d, 4.64 d, 5.22 d |
| 20 | Glutamate | 2.04 m, 2.12 m, 2.34 m, 3.75 m |
| 21 | Glutamine | 2.13 m, 2.44 m, 3.77 t, 6.87 s |
| 22 | Glycerol | 3.55 q, 3.65 q, 3.78 m |
| 23 | Glycine | 3.55 s |
| 24 | Glycolate | 3.93 s |
| 25 | Histamine | 3.00 t, 3.29 t, 7.14 s, 7.89 s |
| 26 | Histidine | 3.14 q, 3.24 q, 3.98 q, 7.10 s, 7.87 s |
| 27 | Imidazole | 7.31 s, 8.28 s |
| 28 | Indole-3-acetate | 3.64 s, 7.15 t, 7.24 t, 7.50 d, 7.62 d, 9.95 s |
| 29 | Isocitrate | 2.50 q, 2.55 q, 2.98 m, 4.05 d |
| 30 | Isoleucine | 0.93 t, 1.00 d, 1.25 m, 1.46 m, 1.97 m, 3.66 d |
| 31 | Lactate | 1.32 d, 4.10 q |
| 32 | Leucine | 0.95 t, 1.70 m, 3.73 q |
| 33 | Methionine | 2.10 t, 2.13 s, 2.19 m, 2.63 t, 3.85 q |
| 34 | Phenylalanine | 3.12 q, 3.28 q, 3.99 q, 7.32 d, 7.37 t, 7.42 t |
| 35 | Proline | 1.96 m, 2.02 m, 2.06 m, 2.34 m, 3.32 m, 3.42 m, 4.12 q |
| 36 | Pyruvate | 2.36 s |
| 37 | Sarcosine | 2.7 s, 3.60 s |
| 38 | Serine | 3.84 q, 3.94 q, 3.98 q |
| 39 | Succinate | 2.39 s |
| 40 | Taurine | 3.25 t, 3.42 t |
| 41 | Threonine | 1.32 d, 3.58 d, 4.25 m |
| 42 | Trimethylamine N-oxide | 3.25 s |
| 43 | Tryptophan | 3.30q, 3.48 q, 4.05 q, 7.19 t, 7.27 t, 7.32 s, 7.53 d, 7.72 d |
| 44 | Tyrosine | 3.04 q, 3.19 q, 3.93 q, 6.89 d, 7.18 d |
| 45 | Uracil | 5.80 d, 7.53 d |
| 46 | Valine | 0.98d, 1.03 d, 2.26 m, 3.60 d |
| 47 | Myo-inositol | 3.27 t, 3.53 q, 3.62 t, 4.06 t |
| VIP Metabolite (D2) | CORT | Control | ta | pb | ||
|---|---|---|---|---|---|---|
| Mean (mM) | SE | Mean (mM) | SE | |||
| 3-Hydroxybutyrate | 0.09 | 0.01 | 0.16 | 0.02 | −4.07 | <0.01 |
| Ethanolamine | 0.18 | 0.02 | 0.35 | 0.03 | −2.14 | 0.04 |
| Formate | 0.48 | 0.08 | 0.16 | 0.21 | 4.81 | <0.01 |
| Glucose | 3.29 | 0.29 | 2.53 | 0.19 | 3.26 | <0.01 |
| Glutamine | 0.24 | 0.04 | 0.15 | 0.10 | 2.79 | 0.01 |
| Indole-3-acetate | 0.06 | 0.01 | 0.12 | 0.02 | −2.69 | 0.01 |
| Lactate | 2.83 | 0.22 | 2.48 | 0.14 | 1.78 | 0.04 |
| Threonine | 0.14 | 0.01 | 0.18 | 0.02 | −3.81 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, A.; Soos, C.; Machin, K. Identification of Metabolomic Biomarkers of Long-Term Stress Using NMR Spectroscopy in a Diving Duck. Metabolites 2022, 12, 353. https://doi.org/10.3390/metabo12040353
Perera A, Soos C, Machin K. Identification of Metabolomic Biomarkers of Long-Term Stress Using NMR Spectroscopy in a Diving Duck. Metabolites. 2022; 12(4):353. https://doi.org/10.3390/metabo12040353
Chicago/Turabian StylePerera, Asha, Catherine Soos, and Karen Machin. 2022. "Identification of Metabolomic Biomarkers of Long-Term Stress Using NMR Spectroscopy in a Diving Duck" Metabolites 12, no. 4: 353. https://doi.org/10.3390/metabo12040353
APA StylePerera, A., Soos, C., & Machin, K. (2022). Identification of Metabolomic Biomarkers of Long-Term Stress Using NMR Spectroscopy in a Diving Duck. Metabolites, 12(4), 353. https://doi.org/10.3390/metabo12040353