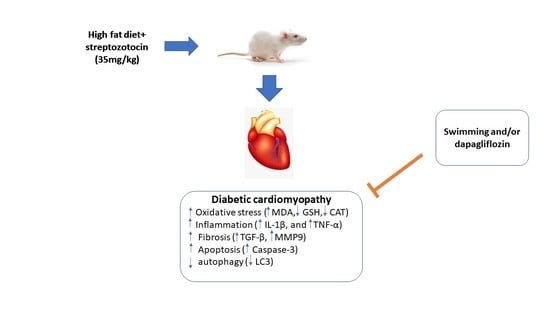
Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Effect of Swimming and/or SGLT2i on Serum Glucose, Insulin, and HOMA Index
2.2. Effect of Swimming and/or SGLT2i on Cardiac Enzymes
2.3. Effect of Swimming and/or SGLT2i on MDA, GSH, and CAT
2.4. Effect of Exercise and/or SGLT2i on TNF-α and IL-1β mRNA Expression in Heart Tissue
2.5. Effect of Swimming and/or SGLT2i on MMP9 and TGFβ mRNA Expression in Heart Tissue
2.6. Effect of Exercise and/or SGLT2i on Histopathological Changes in the Myocardium
2.7. Effect of Exercise or/and SGLT2i on Myocardial Apoptosis
2.8. Effect of Exercise or/and SGLT2i on Autophagy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Development of Experimental Insulin-Resistant Type 2 DM
4.3. Experimental Design
- -
- Control group: non-diabetic normal rats that were treated with 0.5 mL saline and 0.1 mol/L citrate buffer (pH 4.5) at 1 mL/kg, i.p. via gastric gavage.
- -
- Diabetes Mellitus (DM) group: rats with T2DM received 0.5 mL saline/day by oral gavage for 6 weeks.
- -
- DM + exercise/swimming (DM-S) group: T2DM rats were subjected to swimming exercise protocol for 6 weeks.
- -
- DM + SGLT2i, Farxiga (DM-F) group: rats with T2DM received SGLT2i (dapagliflozin, FORXIGA, AstraZeneca, Mississauga, ON, Canada, 1 mg/kg/day) via oral gastric gavage for 6 weeks.
- -
- DM + SGLT2i, Farxiga, and exercise, swimming (DM-FS) group: T2DM rats received dapagliflozin treatment and were subjected to swimming exercise protocol for 6 weeks.
4.4. Drug Preparation and Administration
4.5. Swimming Protocol
4.6. Blood Sampling and Tissue Collection
4.7. Biochemical Study
4.7.1. Measurement of Blood Glucose, Insulin, and Calculation of HOMA Index
4.7.2. Measurement of Cardiac Enzymes
4.8. Measurement of Myocardial Oxidative Stress Markers
4.9. Determination of Tumor Necrosis Factor-α (TNF-α), Interleukin-1B (IL-1β), Matrix Metalloproteinase9 (MMP9), and Tumor Growth Factor-β (TGF-β) Gene Expression by Real-Time PCR (RT-qPCR)
4.10. Histopathological Examination and Fibrosis Evaluation of the Heart Tissues
4.11. Immunohistochemical Staining
4.12. Morphometric Analysis of Immunohistochemical Results
4.13. Statistical Analysis and Data Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.H.; Abel, E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, L.; Song, J.; Zhu, N.; Song, X.; Shi, R.; Ge, G.; Zhang, Y. Tanshinone IIA ameliorates diabetic cardiomyopathy by inhibiting Grp78 and CHOP expression in STZ-induced diabetes rats. Exp. Ther. Med. 2019, 18, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 73. [Google Scholar] [CrossRef]
- Yilmaz, S.; Canpolat, U.; Aydogdu, S.; Abboud, H.E. Diabetic cardiomyopathy; summary of 41 years. Korean Circ. J. 2015, 45, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Kuethe, F.; Sigusch, H.; Bornstein, S.; Hilbig, K.; Kamvissi, V.; Figulla, H.J. Apoptosis in patients with dilated cardiomyopathy and diabetes: A feature of diabetic cardiomyopathy? Horm. Metab. Res. 2007, 39, 672–676. [Google Scholar] [CrossRef]
- Miki, T.; Yuda, S.; Kouzu, H.; Miura, T. Diabetic cardiomyopathy: Pathophysiology and clinical features. Heart Fail Rev 2013, 18, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, A.B.; Gottlieb, R.A. Autophagy in ischemic heart disease. Circ. Res. 2009, 104, 150–158. [Google Scholar] [CrossRef]
- Martinet, W.; Knaapen, M.W.; Kockx, M.M.; De Meyer, G.R. Autophagy in cardiovascular disease. Trends Mol. Med. 2007, 13, 482–491. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Hu, J.; Lin, J.; Wang, T.; Duan, Y.; Man, W.; Feng, J.; Sun, L.; Jia, H. MST1 coordinately regulates autophagy and apoptosis in diabetic cardiomyopathy in mice. Diabetologia 2016, 59, 2435–2447. [Google Scholar] [CrossRef] [Green Version]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E. Sodium–glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef]
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diabetes Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tran, D.; Yang, H.-C.; Nylander, S.; Birnbaum, Y.; Ye, Y. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: An AMPK–mTOR interplay. Cardiovasc. Drugs Ther. 2020, 34, 443–461. [Google Scholar] [CrossRef]
- Ren, D.Y.; Zhang, Y. Cardiovascular benefit of SGLT2 inhibitors in the therapeutics of diabetes mellitus: A close look beyond the horizon. Curr. Drug Targets 2018, 19, 1051–1057. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Oh, C.-M.; Cho, S.; Jang, J.-Y.; Kim, H.; Chun, S.; Choi, M.; Park, S.; Ko, Y.-G. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ. J. 2019, 49, 1183–1195. [Google Scholar] [CrossRef] [Green Version]
- Karjalainen, J.; Peltonen, M.; Vanhala, M.; Korpi-Hyövälti, E.; Puolijoki, H.; Saltevo, J.; Oksa, H.; Saaristo, T.; Tuomilehto, J.; Kujala, U.M. Leisure time physical activity in individuals with screen-detected type 2 diabetes compared to those with known type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 81, 110–116. [Google Scholar] [CrossRef]
- American Diabetes Association. Disclosures: Standards of Medical Care in Diabetes—2019; American Diabetes Association: Alexandria, VA, USA, 2019. [Google Scholar]
- Lavie, C.J.; Johannsen, N.; Swift, D.; Sénéchal, M.; Earnest, C.; Church, T.; Hutber, A.; Sallis, R.; Blair, S.N. Exercise is medicine–the importance of physical activity, exercise training, cardiorespiratory fitness and obesity in the prevention and treatment of type 2 diabetes. Eur. Endocrinol. 2014, 10, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Sakai, K.; Saito, K.; Tsutsui, K.; Yamashita, S.; Kato, N. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes receiving conventional therapy: Clinical implication of the importance of exercise habits during treatment with ipragliflozin. Diabetol. Int. 2017, 8, 275–285. [Google Scholar] [CrossRef]
- Hussein, A.M.; Eid, E.A.; Bin-Jaliah, I.; Taha, M.; Lashin, L.S. Exercise and Stevia Rebaudiana (R) Extracts Attenuate Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Said, E.; Atef, H.; Zaitone, S.A. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: Effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem Biol Interact. 2020, 315, 108897. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, S.Z.T.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complement. Altern. Med. 2017, 17, 156. [Google Scholar]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Depre, C.; Young, M.E.; Ying, J.; Ahuja, H.S.; Han, Q.; Garza, N.; Davies, P.J.; Taegtmeyer, H. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J. Mol. Cell. Cardiol. 2000, 32, 985–996. [Google Scholar] [CrossRef]
- Broderick, T.L.; Poirier, P.; Gillis, M. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes/Metab. Res. 2005, 21, 44–50. [Google Scholar] [CrossRef]
- Novoa, U.; Arauna, D.; Moran, M.; Nuñez, M.; Zagmutt, S.; Saldivia, S.; Valdes, C.; Villaseñor, J.; Zambrano, C.G.; Gonzalez, D.R. High-intensity exercise reduces cardiac fibrosis and hypertrophy but does not restore the nitroso-redox imbalance in diabetic cardiomyopathy. Oxidative Med. Cell. Longev. 2017, 2017, 7921363. [Google Scholar] [CrossRef] [Green Version]
- Oyama, J.-I.; Tanaka, A.; Sato, Y.; Tomiyama, H.; Sata, M.; Ishizu, T.; Taguchi, I.; Kuroyanagi, T.; Teragawa, H.; Ishizaka, N. Rationale and design of a multicenter randomized study for evaluating vascular function under uric acid control using the xanthine oxidase inhibitor, febuxostat: The PRIZE study. Cardiovasc. Diabetol. 2016, 15, 87. [Google Scholar] [CrossRef] [Green Version]
- Al Hroob, A.M.; Abukhalil, M.H.; Hussein, O.E.; Mahmoud, A.M. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed. Pharmacother. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Liu, X.F.; Ma, S.F. Effect of genistein on myocardial fibrosis in diabetic rats and its mechanism. Mol. Med. Rep. 2018, 17, 2929–2936. [Google Scholar] [CrossRef] [Green Version]
- Chengji, W.; Xianjin, F. Exercise protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway in rats. J. Cell. Physiol. 2019, 234, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Andreadou, I.; Efentakis, P.; Balafas, E.; Togliatto, G.; Davos, C.H.; Varela, A.; Dimitriou, C.A.; Nikolaou, P.-E.; Maratou, E.; Lambadiari, V. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: Role of STAT3, mitochondria, and redox aspects. Front. Physiol. 2017, 8, 1077. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Bei, Y.; Lu, Y.; Sun, W.; Liu, Q.; Wang, Y.; Cao, Y.; Chen, P.; Xiao, J.; Kong, X. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell. Physiol. Biochem. 2015, 35, 2159–2168. [Google Scholar] [CrossRef]
- Mahmoud, A.M. Exercise amaliorates metabolic disturbances and oxidative stress in diabetic cardiomyopathy: Possible underlying mechanisms. In Exercise for Cardiovascular Disease Prevention and Treatment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–230. [Google Scholar]
- Joubert, M.; Manrique, A.; Cariou, B.; Prieur, X. Diabetes-related cardiomyopathy: The sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. 2019, 45, 238–247. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Liu, X.; Gao, Q.; Wang, L.; Ma, S. Effect of hydrogen sulfide on oxidative stress and endoplasmic reticulum stress in diabetic cardiomyopathy. Chin. J. Appl. Physiol. 2016, 32, 8–12. [Google Scholar]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signalling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef]
- Olgar, Y.; Turan, B. A sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin comparison with insulin shows important effects on Zn2+-transporters in cardiomyocytes from insulin-resistant metabolic syndrome rats through inhibition of oxidative stress. Can. J. Physiol. Pharmacol. 2019, 97, 528–535. [Google Scholar] [CrossRef]
- Xing, Y.-J.; Liu, B.-H.; Wan, S.-J.; Cheng, Y.; Zhou, S.-M.; Sun, Y.; Yao, X.-M.; Hua, Q.; Meng, X.-J.; Cheng, J.-H. A SGLT2 Inhibitor Dapagliflozin Alleviates Diabetic Cardiomyopathy by Suppressing High Glucose-Induced Oxidative Stress in vivo and in vitro. Front. Pharmacol. 2021, 12, 1756. [Google Scholar] [CrossRef]
- Veeranki, S.; Givvimani, S.; Kundu, S.; Metreveli, N.; Pushpakumar, S.; Tyagi, S.C. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell. Cardiol. 2016, 92, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Kanter, M.; Aksu, F.; Takir, M.; Kostek, O.; Kanter, B.; Oymagil, A. Effects of low intensity exercise against apoptosis and oxidative stress in Streptozotocin-induced diabetic rat heart. Exp. Clin. Endocrinol. Diabetes 2017, 125, 583–591. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Kurdys, J.G.; Muntean, D.M.; Rosca, M.G. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxidants 2019, 30, 375–398. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2017, 94, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Malek, V.; Gaikwad, A.B. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc. Res. 2019, 115, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lahnwong, S.; Chattipakorn, S.C.; Chattipakorn, N. Potential mechanisms responsible for cardioprotective effects of sodium–glucose co-transporter 2 inhibitors. Cardiovasc. Diabetol. 2018, 17, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahnwong, S.; Palee, S.; Apaijai, N.; Sriwichaiin, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc. Diabetol. 2020, 19, 91. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Al-Gayyar, M.M.; Abd El Galil, K.H. Nephroprotective role of dipyridamole in diabetic nephropathy: Effect on inflammation and apoptosis. Life Sci. 2015, 143, 8–17. [Google Scholar] [CrossRef]
- Lorenzo, O.; Picatoste, B.; Ares-Carrasco, S.; Ramírez, E.; Egido, J.; Tuñón, J. Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediat. Inflamm. 2011, 2011, 652097. [Google Scholar] [CrossRef] [Green Version]
- Westermann, D.; Van Linthout, S.; Dhayat, S.; Dhayat, N.; Schmidt, A.; Noutsias, M.; Song, X.-Y.; Spillmann, F.; Riad, A.; Schultheiss, H.-P. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500. [Google Scholar] [CrossRef]
- Adeghate, E.; Singh, J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014, 19, 15–23. [Google Scholar] [CrossRef]
- Petrov, V.V.; Fagard, R.H.; Lijnen, P.J. Stimulation of collagen production by transforming growth factor-β1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002, 39, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Tyagi, N.; Sen, U.; Joshua, I.G.; Tyagi, S.C. Synergism in hyperhomocysteinemia and diabetes: Role of PPAR gamma and tempol. Cardiovasc. Diabetol. 2010, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cheng, J.; Zheng, S.; Zhang, L.; Guo, X.; Zhang, J.; Xiao, X. Physical exercise and its protective effects on diabetic cardiomyopathy: What is the evidence? Front. Endocrinol. 2018, 9, 729. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef] [Green Version]
- Lund, J.; Hafstad, A.D.; Boardman, N.T.; Rossvoll, L.; Rolim, N.P.; Ahmed, M.S.; Florholmen, G.; Attramadal, H.; Wisløff, U.; Larsen, T.S. Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H823–H829. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.-P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload–induced mitochondrial dysfunction and heart failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Packer, M.; Codogno, P. Development of autophagy inducers in clinical medicine. J. Clin. Investig. 2015, 125, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Kuno, A.; Yano, T.; Miki, T.; Oshima, H.; Sato, T.; Nakata, K.; Kimura, Y.; Tanno, M.; Miura, T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol. Rep. 2018, 6, e13741. [Google Scholar] [CrossRef]
- Tanaka, S.; Sugiura, Y.; Saito, H.; Sugahara, M.; Higashijima, Y.; Yamaguchi, J.; Inagi, R.; Suematsu, M.; Nangaku, M.; Tanaka, T. Sodium–glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018, 94, 912–925. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Schumacher, C.A.; Fiolet, J.W.; Kuschma, M.C.; Hollmann, M.W.; Coronel, R.; Weber, N.C.; Zuurbier, C.J. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front. Physiol. 2018, 9, 1575. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.J.; You, Y.H.; Moon, M.K.; Yoon, K.H.; Ahn, Y.B.; Ko, S.H. Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and α-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes. J. Cell. Biochem. 2019, 120, 8534–8546. [Google Scholar] [CrossRef]
- Lee, Y.; Kang, E.-B.; Kwon, I.; Cosio-Lima, L.; Cavnar, P.; Javan, G.T. Cardiac Kinetophagy Coincides with Activation of Anabolic Signaling. Med. Sci. Sports Exerc. 2016, 48, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, J.; Cretoiu, D.; Li, G.; Xiao, J. Exercise-mediated regulation of autophagy in the cardiovascular system. J. Sport Health Sci. 2020, 9, 203–210. [Google Scholar] [CrossRef]
- Elsaid, F.H.; Khalil, A.A.; Ibrahim, E.M.; Mansour, A.; Hussein, A.M. Effects of exercise and stevia on renal ischemia/reperfusion injury in rats. Acta Sci. Pol. Technol. Aliment. 2019, 18, 317–332. [Google Scholar]
- Matthews, D.R.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Freeman, W.M.; Walker, S.J.; Vrana, K.E. Quantitative RT-PCR: Pitfalls and potential. Biotechniques 1999, 26, 112–125. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Rashid, A.; Chrenek, M.A.; Zhang, Q.; Bruce, B.B.; Klein, M.; Boatright, J.H.; Jiang, Y.; Grossniklaus, H.E.; Nickerson, J.M. Analysis of RPE morphometry in human eyes. Mol. Vis. 2016, 22, 898. [Google Scholar]










| Control Group | DM Group | DM-S | DM-F | DM + SF | |
|---|---|---|---|---|---|
| Blood glucose (mg/dL) | 96.00 ± 8.60 | 383.33 ± 17.79 ** | 186.17 ± 9.24 **,## | 152.83 ± 9.91 **,##,$$ | 131.50 ± 3.51 **,##,$$,ØØ |
| Insulin (U/mL) | 11.56 ± 0.941 | 6.82 ± 0.314 ** | 8.13 ± 0.403 **,## | 9.58 ± 0.421 **,##,$$ | 10.90 ± 0.387 **,##,$$,Ø |
| HOMA-IR | 2.72 ± 0.316 | 6.44 ± 0.250 ** | 3.72 ± 0.079 **,## | 3.45 ± 0.464 **,##,$$ | 3.53 ± 0.058 **,##,$$,Ø |
| Control Group | DM Group | DM-S | DM-F | MD + SF | |
|---|---|---|---|---|---|
| CK-MB (U/L) | 20.13 ± 1.50 | 52.08 ± 4.63 ** | 36.23 ± 2.39 **,## | 26.46 ± 2.23 **,##,$$ | 23.55 ± 1.53 *,##,$$ |
| LDH (U/L) | 251.00 ± 7.54 | 965.83 ± 64.99 ** | 519.67 ± 7.11 **,## | 305.00 ± 11.83 *,##,$$ | 276.83 ± 6.55 ##,$$ |
| Gene | Forward Primer | Reverse Primer | Product Length | Reference Sequence |
|---|---|---|---|---|
| IL-1β | GCTATGGCAACTGTCCCTGA | CATCTGGACAGCCCAAGTCA | 136 | NM_031512.2 |
| TNF-α | GGCGTGTTCATCCGTTCTCT | CCCAGAGCCACAATTCCCTT | 133 | NM_012675.3 |
| MMP9 | TGGGCATTAGGGACAGAGGA | TTTCCCCTGTGAGTGGGTTG | 139 | NM_031055.2 |
| TGF-β | CTTTGTACAACAGCACCCGC | CGGGTGACTTCTTTGGCGTA | 94 | NM_021578.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldesoqui, M.; Eldken, Z.H.; Mostafa, S.A.; Al-Serwi, R.H.; El-Sherbiny, M.; Elsherbiny, N.; Mohammedsaleh, Z.M.; Sakr, N.H. Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms. Metabolites 2022, 12, 635. https://doi.org/10.3390/metabo12070635
Eldesoqui M, Eldken ZH, Mostafa SA, Al-Serwi RH, El-Sherbiny M, Elsherbiny N, Mohammedsaleh ZM, Sakr NH. Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms. Metabolites. 2022; 12(7):635. https://doi.org/10.3390/metabo12070635
Chicago/Turabian StyleEldesoqui, Mamdouh, Zienab Helmy Eldken, Sally Abdallah Mostafa, Rasha Hamed Al-Serwi, Mohamed El-Sherbiny, Nehal Elsherbiny, Zuhair M. Mohammedsaleh, and Noha Hammad Sakr. 2022. "Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms" Metabolites 12, no. 7: 635. https://doi.org/10.3390/metabo12070635
APA StyleEldesoqui, M., Eldken, Z. H., Mostafa, S. A., Al-Serwi, R. H., El-Sherbiny, M., Elsherbiny, N., Mohammedsaleh, Z. M., & Sakr, N. H. (2022). Exercise Augments the Effect of SGLT2 Inhibitor Dapagliflozin on Experimentally Induced Diabetic Cardiomyopathy, Possible Underlying Mechanisms. Metabolites, 12(7), 635. https://doi.org/10.3390/metabo12070635