Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions
Abstract
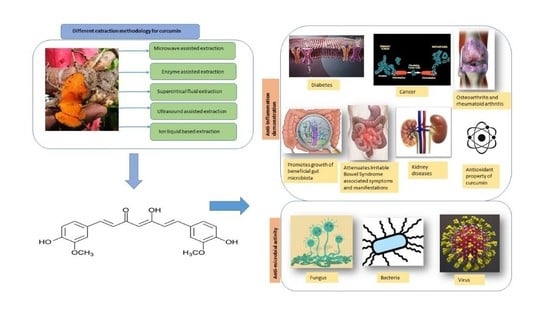
:1. Introduction
1.1. Source of Curcumin
1.2. Chemistry and Bioavailability of Curcumin
1.3. Extraction of Curcumin
2. Methods
3. Anti-Inflammatory Properties of Curcumin
- (1)
- Inflammatory bacterial products such as lipopolysaccharide(LPS) activate the NF-κB pathway to activate NLRP3, leading to Pro-Interleukin-1β (pro-IL-1β) synthesis.
- (2)
- Stimuli such as nigericin, aluminium crystal and monosodium urate crystal lead to NLRP3 activation, subsequently leading to the activation of caspase-1 along with the promotion of proinflammatory cytokines such as IL-1B and IL-18 [39].
3.1. Rheumatoid Arthritis (RA)
3.2. Osteoarthritis (OA)
3.3. Cancer
3.4. Diabetes
| Model | Conc. of Curcumin | Increase | Decrease | No. of Mice/Rats Used | Route of Administration | Reference |
|---|---|---|---|---|---|---|
| Albino Wistar rats with Streptozotocin-induced diabetes | 0.5% of diet; 8 weeks | ATPase activity, PUFA/SFA ratio | Phospholipid, triglyceride, kidney weight, renal lesion progression, renal damage, urine ALT and AST, kidney alkaline and acid phosphatase, glucose-6- phosphatase | 48 | Intraperitoneal | [170] |
| Albino Wistar rats with Streptozotocin-induced diabetes | 300 mg/kg b.w./day for 8 weeks | Creatinine, kidney SOD activity, kidney catalase activity | Glucose, total cholesterol, triglyceride, urea, body weight, kidney lipid peroxidation | 10 | Intraperitoneal | [171] |
| Wistar Rats with Streptozotocin-induced diabetes | 80 mg/kg b.w./day; 45 days | Insulin, SOD, catalase, GPx activity, glutathione-S-transferase | Glucose, lipid peroxidation, TBARS, H2O2 | 24 | Intraperitoneal | [172] |
| Sprague–Dawley rats with Streptozotocin-induced diabetes | 15 and 30 mg/kg b.w./day; 2 weeks | Creatinine clearance, SOD activity, catalase activity | Glucose, creatinine, renal changes, oxidative stress, urine albumin, proteinuria, lipid peroxidation, MDA | N/A | Intraperitoneal | [173] |
| Wistar-NIN rats with Streptozotocin-induced diabetes | 0.01% curcumin; 8 weeks | SOD activity, pancreas catalase activity | Glucose, insulin, TBARS, pancreas SOD activity, glutathione-S-transferase activity | 32 | Intraperitoneal | [174] |
| Sprague–Dawley rats with Streptozotocin induced type 1 diabetes | 50 mg/kg b.w./day; 6 weeks | Albumin, acetyl-histone H3, phospho-histone H3 | Urea, creatinine, HSP-27 protein, p38 protein | 12 | Intraperitoneal | [175] |
| C57/BL6J mice with Streptozotocin-induced diabetes | 7.5 mg/kg b.w./day; 10 h prior to STZ | Insulin, glucose clearance, GLUT2 mRNA | Glucose, IL-16, TNF-α, pancreatic IL-6 | N/A | Intraperitoneal | [176] |
| Wistar rats with Streptozotocin-induced diabetes | 80 mg/kg b.w./day; 45 day | Insulin, SOD activity, CAT activity, GPx activity, glutathione activity | Kidney and liver: morphological changes, oxidative stress, TBARS, HP | 30 | Intraperitoneal | [177] |
| Swiss albino mice with Streptozotocin-induced diabetes | 10 mM; 10 µL/mouse i.p.; 28 days and 106 BMCs, a single injection | Insulin, islet regeneration, SOD activity, catalase activity, GPx activity | Glucose, MDA levels | 40 | Intraperitoneal | [178] |
| Wistar rats with alloxan-induced diabetes | 0.08 mg/kg b.w./day; 21 days | Hemoglobin, glutathione, GPx activity | Glucose, HbA1c, TBARS, SDH activity | 36 | Oral | [179] |
| Wistar rats with alloxan-induced diabetes | 0.1 mg/kg b.w.; 2 h | Glucose | N/A | Oral | [180] |
3.5. Kidney Diseases
3.6. Antioxidant
3.7. Gut Microbiota
3.8. Inflammatory Bowel Disease (IBD)
4. Anti-Microbial
4.1. Antiviral
4.1.1. Human Immunodeficiency Virus (HIV)
4.1.2. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
4.1.3. Influenza A Virus (IAV)
4.1.4. Herpes Simplex Virus (HSV)
4.1.5. Dengue Virus (DENV)
4.1.6. Enterovirus 71 (EV71)
4.2. Antifungal Activity
4.3. Antibacterial
5. Clinical Trials with Curcumin
| Sl No. | Clincal Trial Identifier | Trial Title | No. of Participants | Inclusion Criteria | Year of Completion | Primary Outcome | Clinical Trial No. | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|
| 1. | NCT03085680 | Curcumin and Function in Older Adults | 21 | Aged above 65 years with a CRP level greater than 1.0 mg/dL | 2020 | To examine the effects of dietary supplementation with curcumin on changes in physical function, walking speed (400 m walk test) and grip strength | 2 | 90 days |
| 2. | NCT03211104 | Comparison of Duration of Treatment Interruption with or without Curcumin During the Off Treatment Periods in Patients with Prostate Cancer Undergoing Intermittent Androgen Deprivation Therapy | 107 | Patients with localized prostate cancer or metastatic prostate cancer at the time of diagnosis who received intermittent androgen deprivation therapy (IAD) | 2015 | To determine whether the period from the first interruption of the androgen deprivation therapy to the time when androgen deprivation therapy needs to be retreated differs between the curcumin group and placebo group | NA | 180 days |
| 3. | NCT04012424 | The Effect of Premedication with Curcumin on Post-endodontic Pain | 44 | Patients in the age range of 20–55 years with acute pulpitis | 2020 | Change in postoperative pain after a single endodontic visit | N/A | 2 days |
| 4. | NCT04870060 | Ability of Curcumin to Decrease Cytokines Involved in Mucositis in the Autologous Transplant | 40 | Patients aged 18 years and above with a creatinine clearance greater than 50 mL/min and a serum bilirubin level greater than 2 mg/dl | 2015 | To calculate TNFa, IL-1, IL-6, IL-8, IL-17, TGF-B, IFN-gamma and E2 levels | 2 | 28 days |
| 5. | NCT01543386 | Effects of Curcumin on Vascular Reactivity | 21 | 50- to 70-year-old smokers | 2012 | Changes in brachial flow-mediated dilatation | 2 | 5 days |
| 6. | NCT03568513 | Effect of Curcumin on Gut Microbiota in IBS | 4 | Patients aged 10 to 18 years with diarrhoea-predominant IBS | 2020 | Alterations in gut microbiota | N/A | 56 days |
| 7 | NCT03864783 | The Effect of Curcumin on Liver Fat Content in Obese Subjects | 39 | BMI and haemoglobin greater than 30.0 kg/m2 and 7.5 mmol/L, respectively | 2020 | Curcumin’s effect on steatosis | N/A | 42 days |
| 8. | NCT04044417 | Curcumin-Simvastatin-EDTA in the Treatment of Periodontitis | 30 | Patients aged 25 to 50 years suffering from at least a single posterior 2–3 wall periodontal pocket of depth | 2018 | Reduction in probing depth | 4 | 180 days |
| 9. | NCT04032132 | Curcumin Paste as an Adjunctive Therapy in Periodontitis | 24 | Patients aged 25 to 45 years with at least a single posterior 2–3 wall periodontal defect of pocket depth | 2018 | Evaluate the influence of curcumin paste on the clinical outcomes of the surgical treatment | 4 | 180 days |
| 10. | NCT03746158 | Interindividual Variation in Excretion of Curcumin | 8 | 18–30-year-old healthy adults. | 2019 | Determine the concentration of curcumin and its metabolites in human fecal samples | N/A | 28 days |
| 11. | NCT01179256 | Effect of Supplemental Oral Curcumin in Patients with Atopic Asthma | 16 | Patients aged 18–60 years on low- or medium-dose inhaled corticosteroids | 2010 | Improvement in post-bronchodilator FEV1 | N/A | N/A |
| 12. | NCT01246973 | Oral Curcumin for Radiation Dermatitis in Breast Cancer Patients | 686 | Females aged 21–120 years | 2015 | To measure the Mean Radiation Dermatitis Severity Score | 2 | 42 days |
| 13. | NCT04119752 | Effect of Curcumin on Microvascular Response and Tissue Oxygenation in Older People | 28 | Aged 60– 85 years with two or more risk factors for cardiovascular disease | 2020 | Changes in microvascular reactivity and tissue oxygen saturation. | N/A | 120 min |
| 14. | NCT02255370 | Curcumin Associated with Thiopurin in the Prevention of Post-op Recurrence in Crohn Disease (POPCUR) | 61 | Patients aged 18 years and older with Crohn’s disease | 2018 | Rutgeerts endoscopic score | 3 | 180 days |
| 15. | NCT02298985 | Curcumin Addition to Antipsychotic Treatment in Chronic Schizophrenia Patients | 38 | Patients aged 18–60 years with schizophrenia and a SANS greater than 30 points | 2017 | Positive and Negative Symptoms Scale (PANSS) | 4 | 180 days |
| 16. | NCT01383161 | 18-Month Study of Memory Effects of Curcumin | 46 | Aged 50–90 years with a modified Ischemic score of less than 4 | 2017 | Change from the baseline to 18 months on the Brief Visual Memory Test-Revised | 2 | 540 days |
| 17. | NCT01333917 | Curcumin Biomarkers | 40 | Healthy volunteers aged 40–80 years | 2013 | To understand the changes in gene expression, the ribonucleic acid (RNA) level and apoptosis | 1 | 30 days |
| 18. | NCT01875822 | Open-label Study of Curcumin C-3 Complex in Schizophrenia | 17 | Patients aged 18–65 years with DSMIV schizophrenia and a SANS greater than 30 | 2012 | To understand the change from the baseline negative symptoms: alogia, anhedonia, social withdrawal and lack of motivation | 2 | 112 days |
| 19. | NCT02978339 | A Study Evaluating the Safety and Efficacy of Curcumin in Patients with Primary Sclerosing Cholangitis (PSC) | 15 | Diagnosed with primary sclerosing cholangitis with alkaline phosphatase >1.5× | 2019 | Change in Serum Alkaline Phosphatase (SAP) | 2 | 84 days |
| 20. | NCT04208334 | The Effect of Curcumin for Treatment of Cancer Anorexia-Cachexia Syndrome in Patients with Stage III-IV of Head and Neck Cancer (CurChexia) | 20 | Patients with stage 3–4 head and neck cancer | 2021 | To measure muscle mass | 2 | 60 days |
| 21. | NCT01925287 | Oral Bioavailability of Curcumin from Micronized Powder and Liquid Micelles in Healthy Young Women and Men | 23 | Healthy volunteers with a normal range blood chemistry value | 2013 | To determine total curcumin, demethoxycurcumin and bisdemethoxycurcumin after deconjugation with beta-glucuronidase | 1 | 24 h |
| 22. | NCT02104752 | Curcumin as a Novel Treatment to Improve Cognitive Dysfunction in Schizophrenia | 39 | Volunteers diagnosed with DSM-5 schizophrenia with a corrected vision of at least 20/30 | 2017 | Measurement and treatment research to improve cognition in schizophrenia | 1 | 56 days |
| 23. | NCT02369549 | Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease | 518 | Patients with an eGFR between 15 and 60 mL/min/1.73 m2 with a minimum of 300 mg of protein in urine or with a albumin/creatinine ratio of at least 300 mg | 2020 | Change in albuminuria and the Estimated Glomerular Filtration Rate (eGFR) | 3 | 180 days |
| 24. | NCT02439385 | Avastin/FOLFIRI in Combination with Curcumin in Colorectal Cancer Patients with Unresectable Metastasis | 50 | Colon or rectal cancer patients aged above 19 years with an ASA score of less than 3 | 2019 | To evaluate progression-free survival in colorectal cancer patients | 2 | 730 days |
| 25. | NCT02474953 | A Study to Compare the Pharmacokinetic Profile of a Proprietary Curcumin Formulation to a Comparator Curcumin Product (15PCHB) | 12 | Volunteers aged 18–45 years with a BMI that is 18–29.9 kg/m2(±1 kg/m2) | 2015 | To measure the maximum concentration of curcumin and time until the max concentration of curcumin | 1 | 48 h |
| 26. | NCT04421716 | Testing the Bioavailability of Phytonutrients, Curcumin and Ursolic Acid | 18 | Men aged 18 years or older | 2021 | To evaluate the number, frequency, duration and relation of toxicity events to CURC and UA, the peak serum concentration, the half-life and the time taken to reach the maximum concentration | 1 | 14 days |
| 27. | NCT04258501 | Exploratory Study of Efficacy on Selected Natural Extracts Reducing Post Prandial Blood Glucose Response | 72 | 20–50-year-old healthy individuals with a normal BMI | 2012 | Change in post-prandial blood glucose | NA | 2 h |
| 28. | NCT01035580 | Trial on Safety and Pharmacokinetics of Intravaginal Curcumin | 13 | Volunteers aged 18–45 years currently using a birth control method | 2012 | To reach the maximum selected dose or maximum tolerated dose of intravaginal curcumin without a dose-limiting toxicity | 1 | 14 days |
| 29. | NCT01403545 | Evaluation of Liposomal Curcumin in Healthy Volunteers | 50 | Volunteers in the age group of 18–45 years with a BMI between 18–27 kg/m2 | 2012 | Safety and tolerability of increasing doses of intravenous liposomal curcumin | 1 | 7 days |
| 30. | NCT01225094 | Curcumin to Prevent Complications After Elective Abdominal Aortic Aneurysm (AAA) Repair | 606 | Volunteers aged 18 years or above who have undergone the repair of AAA | 2016 | To measure urine IL-18, NT-ProBNP, hsCRP and serum creatinine | 2 | N/A |
| 31. | NCT01160302 | Curcumin Biomarker Trial in Head and Neck Cancer | 33 | Volunteers aged between 18–90 years willing to undergo tumor biopsies | 2016 | Change in tissue biomarkers and pharmacokinetics of microgranular curcumin | 1 | 28 days |
| 32. | NCT01917890 | Radiosensitizing and Radioprotective Effects of Curcumin in Prostate Cancer | 40 | Aged between 50–80 years with relapsed or treated basal skin cancer and no severe hypertension | 2013 | Biochemical or clinical progression-free survival | N/A | 365 days |
| 33. | NCT00895167 | The Effects of Oral Curcumin on Heme Oxygenase-1 (HO-1) in Healthy Male Subjects (CUMAHS) | 12 | Aged between 18–45 years with a BMI between 18 and 28 kg/m2 | 2009 | The maximal HO-1 mRNA expression and HO-1 protein level in PBMCs | 1 | 48 h |
| 34. | NCT03542240 | Effects of Curcumin Supplementation on Gut Barrier Function in Patients with Metabolic Syndrome | 15 | Waist Circumference—Female: ≥ 88 cm, Male: ≥ 102 cm B. Blood Pressure: ≥ 130/85 mm/Hg. Impaired fasting glucose or HbA1c fasting glucose ≥ 100 mg/dL or HgA1c ≥ 5.7 D. HDL-C—Females: < 50 mg/dL, Males: < 40 mg/dL E. Triglycerides ≥ 150 mg/dL | 2020 | Change in intestinal permeability and intestinal barrier function | N/A | 365 days |
| 35. | NCT00927485 | Use of Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) | 44 | 21–85 years with FAP (with an intact colon or who have had surgery) | 2016 | To determine the number of polyps and the size of polyps | 5 years | |
| 36. | NCT01042938 | Curcumin for the Prevention of Radiation-induced Dermatitis in Breast Cancer Patients | 35 | Females aged 21 years or above with a diagnosis of non-inflammatory breast adenocarcinoma | 2011 | Severity of dermatitis in the radiation treatment site in breast cancer patients | 2 | 49 days |
| 37. | NCT01490996 | Combining Curcumin with FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer (CUFOX) | 41 | 18 years or above, diagnosed with metastatic colorectal cancer and with an ECOG status of 0 or 1 | 2017 | Completion of dose escalation over two cycles of therapy | 2 | 365 days |
| 38. | NCT01975363 | Pilot Study of Curcumin for Women with Obesity and High Risk for Breast Cancer | 29 | Females with an increased risk of breast cancer and a BMI between 25–40 | 2016 | Determine the adherence, tolerability and safety of two doses of nanoemulsion curcumin | N/A | 90 days |
| 39. | NCT01859858 | Effect of Curcumin on Dose Limiting Toxicity and Pharmacokinetics of Irinotecan in Patients with Solid Tumors | 23 | Aged above 19 years with adequate bone marrow, renal and hepatic function and an ECOG status of 0 or 1 | 2016 | Maximum tolerated dose, pharmacokinetics of irinotecan and SN-38 | 1 | 28 days |
| 40. | NCT04103788 | Evaluation of Increased Absorption of a Curcumin Emulsion (CurQ+) in Healthy Volunteers | 10 | Aged between 21 and 75 years | 2018 | Comparative effect of differing serum sample preparation methodologies on curcumin absorption levels | N/A | 6 h |
| 41. | NCT01925547 | Micellar Curcumin and Metabolic Syndrome Biomarkers | 42 | Total cholesterol > 5.2 mmol/L, LDL cholesterol > 3.4 mmol/L, Triglyceride > 2.26 mmol/L, CRP > 2 mg/L | 2014 | To measure the serum CRP level | 2 | 42 days |
| 42. | NCT01330810 | Curcumin Pharmacokinetics | 12 | Aged between 16 and 65 years with a BMI in the range of 18–30 kg/m2 | 2012 | To measure the AUC, Cmax, Tmax, Ke, T1/2, Vd and bioequivalence of tissue curcumin concentration | 1 | 48 h |
| 43. | NCT02908152 | Curcumin Supplement in Nonalcoholic Fatty Liver Patients | 50 | Patients diagnosed with type 2 diabetes with a CAP score greater than 263 | 2017 | To measure hepatic steatosis | 2 | 72 days |
| 44. | NCT01201694 | Phase I Study of Surface-Controlled Water Soluble Curcumin (THERACURMIN CR-011L) | 28 | Patients aged 13 or older with an ECOG status of 3 or better and normal organ and marrow function | 2014 | To measure the Maximum Tolerated Dose (MTD) of surface-controlled water-dispersible curcumin | 1 | 28 days |
| 45. | NCT04028739 | Theracurmin vs. Curcumin Bioavailability Study | 24 | Healthy adults aged 19–60 years with a BMI of 18–30 kg/m2 | 2019 | To compare the bioavailability of curcumin in healthy adults | NA | 12 h |
| 46. | NCT03795792 | Oral Curcumin Administration to Remit Metabolic Syndrome | 105 | Men and women aged 20–55 years old with metabolic syndrome according to the ATP III criteria | 2019 | Remission of metabolic syndrome (≤2 components according to the ATP III criteria) | NA | 3 months |
| 47. | NCT00528151 | A Randomized, Double-blind, Placebo-controlled Trial of Curcumin in Leber’s Hereditary Optic Neuropathy (LHON) | 70 | Aged 8 years or older with Leber’s hereditary optic neuropathy | 2007 | Visual outcome | 3 | 1 year |
| 48 | NCT00889161 | Curcumin in Pediatric Inflammatory Bowel Disease | 11 | 8–18-year-old patients with IBD who have been on IBD medication for 3 months | 2010 | To determine the tolerability of curcumin in pediatric patients with inflammatory bowel disease | 1 | 9 weeks |
| 49 | NCT01514266 | Effect of Curcumin on Lung Inflammation | 57 | ≥45-year-old patients with COPD and a stable clinical course | 2010 | Change in sputum dysplasia | NA | 3 months |
| 50 | NCT00779493 | Curcumin (Tumeric) in the Treatment of Irritable Bowel Syndrome: A Randomized-Controlled Trial (CuTIBS) | 17 | ≥18-year-old patients who conform to the Rome III criteria | 2009 | The primary outcome will be defined as at least a 50% reduction in the irritable bowel severity score (IBSS) | 4 | 6 months |
| 51 | NCT03329781 | Modulation of Endotoxaemia Via Curcumin Intake in Healthy Overweight Adults (ENDOCUR) | 16 | 18–45-year-old healthy individuals with a BMI ≥ 25 kg/m2 | 2018 | Level of endotoxin in plasma | NA | 21 days |
| 52 | NCT00094445 | Trial of Curcumin in Advanced Pancreatic Cancer | 50 | ≥45-year--old patients with unresectable adenocarcinoma of the pancreas | 2014 | 6-month participant survival | 2 | 6 months |
| 53 | NCT01750359 | Efficacy and Safety Curcumin in Depression | 40 | 20–60-year-old patients with a major depressive disorder | 2011 | Change in Hamilton Depression Rating Scale and Montgomery–Asberg Depression Rating Scale | 4 | 6 weeks |
| 54 | NCT00181662 | Pharmacokinetics of Curcumin in Healthy Volunteers | 6 | ≥45year-old healthy female individuals | 2007 | Curcumin pharmacology | NA | NA |
| 55 | NCT03598205 | Curcumin and Intravitreal Dexamethasone in Diabetic Macular Edema (DIABEC) | 72 | 18–90-year-old patients with significant diabetic macular edema and a central retinal thickness of >300 microns | 2019 | Mean difference in central retinal thickness from baseline to 6 months | NA | 6 months |
| 56 | NCT00641147 | Curcumin in Treating Patients with Familial Adenomatous Polyposis | 44 | 18–85-year-old patients with familial adenomatous polyposis | 2016 | The average number of polyps in the placebo arm at the end of the study is compared to the average in the curcumin arm | 2 | 12 months |
| 57 | NCT04385979 | Curcumin and Nanocurcumin in Oral Aphthous Ulcer | 48 | Patients with minor and recurrent aphthous ulcers with 48 h | 2020 | Wound size and pain score | NA | 1 week |
| 58 | NCT01320436 | Curcumin + aminosalicylic Acid (5ASA) Versus 5ASA Alone in the Treatment of Mild to Moderate Ulcerative Colitis | 50 | 18–70-year-old patients with confirmed diagnosis of ulcerative colitis on a stable dose of ulcerative colitis medication | 2014 | The percentage of patients who achieve clinical remission compared between the two study arms | 3 | 4 weeks |
| 59 | NCT03072992 | “Curcumin” in Combination with Chemotherapy in Advanced Breast Cancer | 150 | 18–75-year-old female patients diagnosed with breast carcinoma and adequate organ function | 2019 | Objective response rate, assessed with the Modified Response Evaluation Criteria in Solid Tumours (RECIST) | 2 | 24 weeks |
| 60 | NCT00113841 | Curcumin (Diferuloylmethane Derivative) With or Without Bioperine in Patients with Multiple Myeloma | 42 | Patients with multiple myeloma and adequate organ function | 2009 | Percent change of NF-kB protein expression in peripheral blood mononuclear cells | NA | 4 weeks |
| 61 | NCT01909037 | Exploratory non comparative Study to Evaluate the Efficacy of Highly Bioavailable Curcumin (Flexofytol) in Patients with Knee Osteoarthritis | 22 | 45–80-year-old patients with osteoarthritis and a symptomatic knee for more than 6 months who can avoid using analgesics during the study | 2012 | Change in the serum levels of biomarkers of cartilage metabolism and inflammation | 1 | 84 days |
| 62 | NCT00365209 | Phase II A Trial of Curcumin Among Patients with Prevalent Subclinical Neoplastic Lesions (Aberrant Crypt Foci) | 44 | ≥40-year-old patients with a >3 pack-year smoking history | 2011 | Change in prostaglandin E2 (PGE2) values found in rectal aberrant crypt foci (ACF) tissue | 2 | 30 days |
| 63 | NCT02494141 | Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults With ADPKD | 68 | 6–25-year-old patients with an ADPKD diagnosis and normal renal function | 2021 | Change in brachial artery flow-mediated dilation (FMD-BA) and aortic pulse-wave velocity (aPWV) | 4 | 12 months |
| 64 | NCT04378972 | Anti-inflammatory Effect of Curcumin, Homotaurine, Vitamin D3 on Human Vitreous in Patients with Diabetic Retinopathy | 25 | ≥18-year-old patients with diabetic retinopathy requiring vitrectomy | 2019 | Analyze human vitreous samples’ pro-inflammatory cytokines | NA | 7 days |
| 65 | NCT04972045 | Bioavailability of Curcumin Capsules in Healthy Adult Subjects | 12 | 18–55-year-old healthy subjects with a BMI of 18–28 kg/m2 | 2021 | Measure Peak Plasma Concentration, area under the curve, Tmax and bioavailability | 1 | 3 days |
| 66 | NCT01489592 | Effect of Curcumin on Iron Metabolism in Healthy Volunteer (CURHEP) | 18 | 18–35-year-old healthy adults with a BMI of 18–25 and no HFE mutation | 2012 | Maximal variation of the serum hepcidin level after the oral administration of curcumin | 2 | 48 h |
| 67 | NCT01964846 | Effect of Antioxidant Intake on Cardiovascular Risk | 22 | 45–70-year-old healthy patients with a stable weight | 2015 | Change in the blood levels of anti- and pro-inflammatory markers | NA | 2 weeks |
| 68 | NCT02100423 | Curcumin and Cholecalciferol in Treating Patients with Previously Untreated Stage 0-II Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma | 35 | ≥18-year-old patients with a CLL or SLL diagnosis and adequate organ function | 2018 | Overall response rate (biologic response rate + complete response [CR] + partial response [PR]) based on NCI-WG (for CLL) and the Cheson criteria (for SLL | 2 | 2 years |
| 69 | NCT03530436 | Comparison of Curcumin Bioavailability | 12 | 18–35-year-old healthy individuals | 2018 | Pharmacokinetics of curcuminoids (curcumin, demethoxycurcumin, bisdemethoxycurcumin) at different time frames | NA | 24 h |
| 70 | NCT02529982 | Curcumin Supplementation and Patients with Type 2 Diabetes | 44 | 44–65-year-old patients with type 2 Diabetes Mellitus with a BMI of 18.5–30 kg/m2 | 2016 | Fasting blood sugar, insulin, HbA1c, homeostatic model assessment of insulin resistance and change in pancreatic B-cell function | NA | 10 weeks |
| 71 | NCT03066791 | Turmeric and Curcumin on Sebum Production | 30 | 18–50-year-old healthy individuals | 2017 | Sebum production | NA | 8 weeks |
| 72 | NCT01514370 | Dietary Supplement of Curcumin in Subjects with Active Relapsing Multiple Sclerosis Treated With Subcutaneous Interferon Beta 1a (CONTAIN) | 80 | 18–60-year-old patients with multiple sclerosis under the treatment of IFN beta-1a for 6–12 months | 2016 | Number of subjects with active (new or enlarging) T2 lesions, as assessed by magnetic resonance imaging (MRI) at Month 12 | 2 | 24 months |
| 73 | NCT00475683 | Curcumin for Prevention of Oral Mucositis in Children Chemotherapy | 8 | 5–30-year-old patients diagnosed with cancer who received doxorubicin containing chemotherapy | 2010 | Measured change of an objective measurement of oral mucositis | 3 | 6 weeks |
| 74 | NCT00164749 | A Pilot Study of Curcumin and Ginkgo for Treating Alzheimer’s Disease | 36 | ≥50-year-old patients of Chinese ethnicity with a progressive decline in memory ≥6 months | 2006 | Measured change in the isoprostane level in plasma and the A-beta level in serum | 2 | 6 months |
| 75 | NCT02152475 | Photodynamic Therapy (PDT) for Oral Disinfection | 30 | 20–35-year-old healthy adults who do not perform any oral hygiene | 2013 | Microbiological analysis by the total number of colony-forming units | 1 | 2 h |
| 76 | NCT01831193 | Effect of Oral Supplementation with Curcumin (Turmeric) in Patients with Proteinuric Chronic Kidney Disease | 120 | 18–70-year-old patients diagnosed with proteinuric chronic kidney disease taking ARB or ACEi | 2014 | Change in proteinuria | 3 | 8 weeks |
| 77 | NCT02556632 | Prophylactic Topical Agents in Reducing Radiation-Induced Dermatitis in Patients with Non-inflammatory Breast Cancer (Curcumin-II) | 191 | ≥21-year-old patients diagnosed with non-inflammatory breast cancer or carcinoma in situ who are undergoing radiation therapy | 2016 | Measured mean Radiation Dermatitis Severity (RDS) score, incidence of moist sesquamation and change in the severity of skin reactions using RDS | 2 | 1 week post-radiation chemotherapy |
| 78 | NCT04465851 | Effect of Ferrous iROn and cUrcumin sTatus on Inflammatory and Neurotrophic markErs (Fe-ROUTINE) | 155 | 18–40-year-old healthy individuals | 2020 | To assess the influence of curcumin administration on ferrous iron supplementation-associated inflammation | NA | 42 days |
| 79 | NCT00192842 | Gemcitabine With Curcumin for Pancreatic Cancer | 17 | ≥18-year-old patients suffering from advanced or metastatic pancreatic adenocarcinoma with no prior therapy | 2010 | time to tumor progression | 2 | NA |
| 80 | NCT00099710 | Curcumin in Patients with Mild to Moderate Alzheimer’s Disease | 33 | ≥50-year-olds with a diagnosis of Alzheimer’s disease | 2007 | Measured safety and tolerability of curcumin | 2 | 12 months |
| 81 | NCT01712542 | Curcumin Bioavailability in Glioblastoma Patients | 15 | ≥18-year-old patients with glioblastoma | 2013 | Measured concentration of curcumin in glioblastoma | NA | At time of tumor resection |
| 82 | NCT01022632 | Effect of Curcumin as Nutraceutical in Patients of Depression | 60 | 18–65-year-old patients with a diagnosis of depression | 2010 | Measured response and mean change in the Hamilton Depression Rating Scale (HAM-D17) | NA | 6 weeks |
| 83 | NCT03144882 | Evaluation of Curcumin’s Effect on Inflammation in Hemodialysis Patients | 71 | ≥18-year-old clinically stable patients receiving hemodialysis | 2017 | Measured mean Interleukin-6 levels | NA | 1 year |
| 84 | NCT03141918 | Effect of Supplementation of Bioactive Compounds on the Energy Metabolism of People Living With HIV/AIDS | 20 | 18–70-year-old patients with HIV receiving antiretroviral therapy ≥6 months | 2017 | Measuring the oxidation of energetic substrates; evaluation at rest | NA | 10 days |
| 85 | NCT01740323 | Phase II Study of Curcumin vs. Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy | 30 | ≥18-year-old female patients undergoing breast radiotherapy | 2018 | Measured change in NF-kB DNA binding, Plasma TNF-alpha, sTNFR2, IL-1ra, IL-6 and CRP | 2 | 6 weeks after the completion of radiotherapy |
| 86 | NCT04107987 | Berberine, Curcumin, Inositol, Banaba and Chromium Picolinate in Patients with Fasting Dysglycemia | 148 | 18–75-year-old patients with impaired fasting glucose who are not on treatment | 2019 | Measured progression of dysglycemia | 3 | 3 months |
| 87 | NCT00027495 | Curcumin for the Prevention of Colon Cancer | NA | ≥18-year-old healthy individuals | 2007 | To determine the pharmacokinetics and measure the Maximum Tolerated Dose (MTD) | 1 | 72 h |
| 88 | NCT04723849 | Efficacy Evaluation of a Mixed Compound of Antioxidants in Terms of Endothelium Damage/Function in Pediatric Subjects with Obesity. (OBELIX) | 48 | 6–17-year-old patients with a BMI > 95% for their age based on the CDC standard | 2020 | To test the effects of a mixed compound including curcumin on endothelium in a cohort of pediatric subjects with obesity | NA | 6 months |
| 89 | NCT00768118 | A Nutritional Supplement Capsule Containing Curcumin, Green Tea Extract, Polygonum Cuspidatum Extract, and Soybean Extract in Healthy Participants | 11 | ≥18-year-old healthy individuals | 2008 | Measure the magnitude of change in the blood lymphocyte NF-kB level | NA | 15 days |
| 90 | NCT02017353 | Effect of Curcumin Addition to Standard Treatment on Tumour-induced Inflammation in Endometrial Carcinoma | 7 | ≥18-year-old female patients with endometrial carcinoma and no life-threatening metastases | 2016 | Measured change in the inflammatory markers in peripheral blood from the baseline | 2 | 21 days |
| 91 | NCT00792818 | The Efficacy and Safety of Curcuma Domestica Extracts and Ibuprofen in Knee Osteoarthritis | 367 | 50–75-year-old patients diagnosed with primary osteoarthritis | 2012 | Measured change in mean Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain subscale | 3 | 12 months |
| 92 | NCT03290417 | Correlative Analysis of the Genomics of Vitamin D and Omega-3 Fatty Acid Intake in Prostate Cancer | 37 | Patients diagnosed with prostate cancer who are on active surveillance | 2019 | Measured gene expression of very low and low-risk prostate cancer patients on active surveillance | NA | 12 months |
| 93 | NCT00525421 | A Clinical Study of Curcuminoids in the Treatment of Oral Lichen Planus | 20 | ≥21-year-old patients diagnosed with lichen planus | 2009 | Measured percent change from the baseline to two weeks in the symptoms and signs of oral lichen planus | 2 | 2 weeks |
| 94 | NCT02337192 | Antimicrobial Photodynamic Therapy Applied in Orthodontic Patients. | 24 | 18–50-year-old healthy individuals with fixed orthodontic treatment | 2014 | Microbiological analysis by the total number of colony-forming units (CFU) | 1 | 1 h |
| 95 | NCT01288859 | Physiological Effects of New Polyphenol-enriched Foods in Humans | 10 | 18–45-year-old healthy individuals | 2011 | Measured serum polyphenol concentrations, urinary excretion of total polyphenols and the number of total fecal polyphenols | NA | 24 h |
| 96 | NCT01029327 | Effects of Curcumin on Postprandial Blood Glucose, and Insulin in Healthy Subjects | 15 | ≥18-year-old healthy individuals | 2009 | To study the effect of curcumin on the postprandial blood glucose and plasma concentrations of insulin | NA | NA |
| 97 | NCT02815475 | Turmeric Anti-Inflammatory and Cell-Damage Trial (TACT) | 90 | 18–80-year-old healthy individuals | 2016 | Measured change from baseline DNA methylation analyses and baseline oxidative stress determination | NA | 6 weeks |
| 98 | NCT03769857 | NEM® + BIOCURC® Versus Placebo in Exercise-induced Joint Pain, Stiffness, & Cartilage Turnover in Healthy Men & Women | 84 | 40–75-year-old healthy adults with no diagnosis of joint arthritis | 2019 | Measured exercise-induced cartilage turnover | NA | 2 weeks |
| 99 | NCT03621865 | A Comparative Pharmacokinetic Study to Evaluate the Ability of a New Formulation to Enhance Curcuminoids Bioavailability (TURBIO) | 30 | 18–45-year-old healthy individuals with a normal BMI and a stable weight | 2018 | Measured dose-normalized AUC of total curcuminoids plasmatic concentration | NA | 24 h |
| 100 | NCT03289832 | Effect of Orally Delivered Phytochemicals on Aging and Inflammation in the Skin | 25 | 18–70-year-old healthy individuals willing to avoid sun exposure and follow a diet | 2019 | Measured change in erythema 1, 2 and 3 Days after UV exposure | NA | 10 days |
| 101 | NCT03140657 | The Effects of Nanocurcumin on Treg Cells and Th17 Cells Responses in Ankylosing Spondylitis Patients | 24 | 23–46-year-old patients with a diagnosis of ankylosing spondylitis | 2018 | Assessments of ankylosing spondylitis signs and symptoms (BASDI) | 2 | 4 months |
| 102 | NCT03192059 | Study of Pembrolizumab, Radiation and Immune Modulatory Cocktail in Cervical/Uterine Cancer (PRIMMO) | 43 | ≥18-year-old female patients with endometrial, cervical or uterine malignancy refractory to treatment | 2021 | Measured efficacy (objective response rate) at week 26 according to the immune-related response criteria (irRC) | 2 | 156 weeks |
| 103 | NCT03530787 | Cosmetic Effects of Topical Acetyl Zingerone | 31 | 30–60-year-old healthy individuals | 2018 | Measured change in wrinkle appearance and skin pigmentation | NA | 8 weeks |
| 104 | NCT03493997 | Multicentre International STudy for the Prevention with Ialuril® of Radio-induced Cystitis (MISTIC) | 100 | ≥18-year-old male patients who planned to receive primary therapy for prostate cancer | 2018 | Measured rate of patients who stopped treatment with intravesical or oral ialuril due to intolerance or adverse events | 2 | 12 months |
| 105 | NCT04849182 | Vertistop® D and Vertistop® L in Preventing Recurrence of High-recurrence BPPV | 128 | 18–85-year-old patients with benign paroxysmal positional vertigo (BPPV) | 2020 | Measured number of BPPV recurrences in patients supplemented with Vertistop D | NA | 6 months |
| 106 | NCT02099890 | The Effect of Diet on Chronic Inflammation and Related Disorders Following Spinal Cord Injury | 20 | ≥18-year-old patients with a spinal cord injury | 2015 | Measured change from the baseline in the nerve conduction velocity of somatic nerves at 3 and 6 months | 3 | 6 months |
| 107 | NCT03483376 | aPDT for the Remediation of Dental Black Stain | 30 | ≥12-year-old patients with a dental black stain in at least two teeth | 2020 | Area and depth of color of the black stain | NA | 6 months |
| 108 | NCT00235625 | Curcuminoids for the Treatment of Chronic Psoriasis Vulgaris | 12 | 18–75-year-old patients with chronic plaque-type psoriasis | 2007 | Physicians Global Assessment (PGA) of change | 2 | 16 weeks |
| 109 | NCT04382040 | A Phase II, Controlled Clinical Study Designed to Evaluate the Effect of ArtemiC in Patients Diagnosed With COVID-19 | 50 | ≥18-year-old patients with a diagnosis of SARS-CoV-2 infection who are hospitalized and are in stable condition | 2020 | Time to clinical improvement, defined as a national Early Warning Score 2 (NEWS2) of ≤ 2, maintained for 24 h, and measurement of adverse events | 2 | 2 weeks |
| 110 | NCT03150966 | The Immunomodulatory Effects of Oral Nanocurcumin in Multiple Sclerosis Patients | 41 | 18–65-year-old patients who are diagnosed with multiple sclerosis | 2017 | Measurement of the Expanded Disability Status Scale (EDSS) | 2 | 6 months |
| 111 | NCT02442453 | Effect of Scaling and Root Planing Along with Topical Application of Commercially Available Curcuma Longa Gel on Superoxide Dismutase and Malondialdehyde Levels in Saliva of Chronic Periodontitis Patients | 100 | 30–55-year-old healthy individuals with chronic generalized periodontitis | 2014 | Measurement of the superoxide dismutase antioxidant enzyme levels in the saliva of chronic periodontitis subjects | 4 | 1 month |
| 112 | NCT02909621 | Evaluation of FLEXOFYTOL® Versus PLACEBO (COPRA) | 150 | 45–80-year-old patients with knee osteoarthritis | 2017 | Measuring the variation in the serum levels of the sColl2-1 biomarker between T0 and T3 by specific immunoassays and the variation in the global assessment of disease activity by the patient using a visual analogue scale (VAS) | NA | 6 months |
| 113 | NCT04439981 | Curcuma Extract Beneficial for Muscle Damage | 20 | 14–18-year-old healthy male individuals | 2019 | Change in lactic acid, Hb, IL-6 and creatinine kinase | NA | 21 days |
| 114 | NCT02251678 | Evaluate the Effect of Elimune Capsules | 21 | ≥18-year-old patients with plaque psoriasis with or without arthritis | 2015 | Individual subject serum levels of biomarkers (CRP, TNFa, IL-6, IL-12) | 1 | 28 days |
| 115 | NCT04633551 | Vascular Inflammation and Anti-inflammatory Supplements After Adverse Pregnancy Outcomes (VIA) | 8 | 18–45-year-old female patients who had a singleton pregnancy of < 3 years complicated by an adverse pregnancy outcome (APO) | 2021 | Measurement of blood pressure, arterial stiffness, augmentation index and endothelial function | NA | 1 month |
| 116 | NCT02834078 | Effect of BGG on Glucose Metabolism and Other Markers of Metabolic Syndrome (Glucogold) | 126 | 20–60-year-old patients with a BMI ≥ 25 suffering from pre-diabetes or early diagnosed diabetes | 2016 | Measured change in the oral disposition index and HbA1c | NA | 84 days |
| 117 | NCT04149639 | A Study Investigating the Effectiveness of a LifeSeasons NeuroQ Supplement with Lifestyle Changes to Improve Cognitive Function in Healthy Adults Who Have One or More Risk Factors for Cognitive Decline | 40 | ≥45-year-old patients with risk factors for cognitive decline | 2020 | Measured change in cognition as assessed by the change in the Neurocognitive Index (NCI) score from the CNS-Vital Signs (CNS-VS) panel | NA | 135 days |
| 118 | NCT01716637 | Short Term Efficacy and Safety of Perispinal Administration of Etanercept in Mild to Moderate Alzheimer’s Disease | 12 | 60–85-year-old patients with a diagnosis of Alzheimer’s disease | 2016 | Difference in the effects of the treatment for 6 weeks with etanercept + nutritional supplements versus nutritional supplements alone on the Mini-Mental Status Examination (MMSE) score | 1 | 16 weeks |
| 119 | NCT01752868 | Can Fish Oil and Phytochemical Supplements Mimic Anti-Aging Effects of Calorie Restriction? | 56 | 40–60-year-old patients with a BMI of 21–30 kg/m2 who are sedentary to moderately active | 2012 | Carotid-femoral pulse wave velocity | NA | 6 months |
| 120 | NCT00799630 | Effects of Nutraperf Consumption in Runners | 14 | 18–46-year-old healthy male distance runners | 2008 | Measurement of different metabolic parameters (heart rate, oxygen consumption, respiratory quotient, ventilation, glycemia, lactatemia) on central and peripheral fatigue and on cognitive parameters | NA | NA |
| 121 | NCT04765527 | Turmeric and Exercise-Induced Muscle Damage and Oxinflammation | 53 | 18–50-year-old healthy individuals who are willing to exercise | 2021 | Measuring a change in the serum concentration of creatine kinase | NA | 4 days |
| 122 | NCT02413099 | The Efficacy and Safety of New Herbal Formula (KBMSI-2) in the Treatment of Erectile Dysfunction | 44 | 18–40-year-old male patients with a history of erectile dysfunction | 2013 | Measuring a change in the EF domain scores of the IIEF questionnaire from the baseline | 4 | 8 weeks |
| 123 | NCT01906840 | Role of Turmeric on Oxidative Modulation in ESRD Patients | 48 | ≥18-year-old patients who undergo regular dialysis | 2012 | Measuring the effects of turmerics on oxidative stress markers | 2 | 8 weeks |
| 124 | NCT01646047 | Diabetes Visual Function Supplement Study (DiVFuSS) | 70 | ≥18-year-old patients with a ≥5-year history of diabetes mellitus | 2014 | Measuring changes in visual function | NA | 6 months |
| 125 | NCT02369536 | Efficacy of a Natural Components Mixture in the Treatment of non-Alcoholic Fatty Liver Disease (NAFLD) (NUTRAFAST) | 126 | 18–80-year-old patients with non-alcoholic fatty liver disease (NAFLD) | 2016 | Hematic levels of hepatic enzymes AST, ALT and GGT | NA | 3 months |
| 126 | NCT02088307 | Study of the Cardiovascular Vitamin, CardioLife | 21 | 18–90-year-old patients with cardiovascular disease | 2016 | Change in blood pressure | NA | 6 months |
| 127 | NCT05089318 | Evaluation of Flexofytol® PLUS in Hand Osteoarthritis. | 239 | ≥45-year-old patients with hand arthritis and a regular use of analgesia | 2021 | Pain using a Visual Analog Scale (VAS) | NA | 84 days |
| 128 | NCT03482401 | Disposition of Dietary Polyphenols and Methylxanthines in Mammary Tissues from Breast Cancer Patients (POLYSEN) | 40 | ≥18-year-old patients diagnosed with breast cancer | 2019 | Quantification of dietary polyphenols and methylxanthines in breast tissues | NA | 24 months |
| 129 | NCT04890704 | Curcuminoids and Contrast-induced Acute Kidney Injury | 96 | 18–80-year-old patients undergoing elective CAG with a stable eGFR of 15–60 mL/min/1.72 m2 | 2019 | The incidence of CI-AKI development between the addition of curcuminoids to the standard protocol and the standard protocol alone in patients who underwent CAG | 1 | 48 h |
| 130 | NCT00219882 | Safety Study of Orally Administered Curcuminoids in Adult Subjects with Cystic Fibrosis (SEER) | 11 | 18–40-year-old patients who suffer from cystic fibrosis (homozygous for the ΔF508 CFTR genotype) | 2006 | Safety and tolerability of 14 days of treatment with orally administered curcuminoids, as assessed by adverse events, laboratory parameters and spirometry | 1 | 14 days |
| 131 | NCT04844658 | COVID-19, Hospitalized, PatIents, Nasafytol | 51 | ≥18-year-old patients with a recent hospitalization due to SARS-CoV-2 | 2021 | Improvement of the patient’s clinical condition based on the WHO ordinal outcomes score, the duration of hospitalization, mortality, fever, oxygen therapy, adverse events and several blood parameters | NA | 14 days |
| 132 | NCT03065504 | Turmeric and Turmeric-containing Tablets and Sebum Production | 30 | 18–50-year-old healthy individuals | 2017 | Change in facial sebum production | NA | 4 weeks |
| 133 | NCT04281758 | Comparison of Plasma Caffeine Concentration After Oral Consumption of Caffeinated Beverages with Varied Bioactive Compounds in Healthy Volunteers | 16 | 18–55-year-old healthy individuals willing to avoid caffeine and alcohol for a period of time | 2020 | Incremental area-under-the-concentration-curve (iAUC) | 1 | 210 min |
| 134 | NCT04258501 | Exploratory Study of Efficacy on Selected Natural Extracts Reducing Post Prandial Blood Glucose Response | 72 | 20–50-year-old healthy individuals with a normal BMI | 2012 | Change in post-prandial blood glucose | NA | 2 h |
6. Limitations
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Acetyl coenzyme |
| ADPC | Androgen-dependant prostate cancer |
| AIPC | Androgen-independent prostate cancer |
| AKT | Protein kinase B (also called PKB) |
| ALT | Alanine transaminase |
| AMPK | AMP-activated protein kinase |
| AP | Activator protein |
| AST | Aspartate aminotransferase |
| BAFF | B cell activating factor |
| BCL | B cell lymphocyte |
| Bcl | B cell lymphoma |
| Bcl-xL | B cell lymphoma-extra large |
| CD | Cluster differentiation |
| CHOP | Cytoxan hydroxydaunorubicin oncovin prednisone |
| Coll2 | Collagen |
| COX | Cyclooxygenase |
| Cur-CQD | Curcumin carbon quantom dots |
| CXCL | Chemokine (C-X-C motif) ligand |
| DHA | Docosahexaenoic acid |
| DYRK | Dual specificity tyrosine phosphorylation-regulated kinase |
| EAE | Enzyme-assisted extraction |
| EBP | Enhancer binding protein |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular-regulated kinase |
| ERK | Extracellular-regulated kinase |
| FFA | Free fatty acid |
| FOX | Forkhead box protein |
| FtsZ | Filamenting temperature sensitive mutant Z |
| GST | Glutathione S- transferase |
| Hp | Haptoglobin |
| HSP | Heat shock protein |
| IFN | Interferon |
| IKBα | Inhibitor of kappa light chain gene enhancer in B cells |
| IL | Interlekin |
| ILE | Ionic liquid-based extraction |
| IL | Interleukin |
| iNOS | Inducible nitric oxide syntase |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| JNK | Jun N-terminal kinase |
| LPS | Lipopolysaccharides |
| MAE | Microwave-assisted extraction |
| MAPK | Mitogen-activated protein kinase |
| MCP | Methyl-accepting chemotaxis protein |
| M-CSF | Macrophage colony stimulating factor |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| MIP | Macrophage inflammatory protein |
| MMP | Matrix metalloproteinase |
| MPA | Medroxyprogesterone acetate |
| MTOR | Mammalian target of rapamycin |
| MyD | Myeloid differentiation |
| NF-κkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP | Nod-like receptor protein |
| NOD | Nucleotide oligomerization domain |
| Nrf | Nuclear respiratory factor |
| NSAID | Non-steroidal anti-inflammatory drugs |
| OC | Oleocanthal |
| ODC | Ornithine decarboxylase |
| PECAM | Platelet endothelial cell adhesion molecule |
| PLGA | Poly(D,L-Lactic-co-glycolic acid) |
| PPAR | Peroxisome proliferator-activated receptors |
| Rac 1 | Rass-related C 3 botulinum toxin substrate 1 |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| RANTES | Regulated on activation, normal T cell expressed and secreted |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SDH | Succinate dehydrogenase |
| SFE | Supercritical fluid extraction |
| Shh | Sonic hedgehog protein |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TBARs | Thiobarbituric acid reactive substances |
| TLR | Toll-like receptor |
| TLR | Toll-like Receptor |
| TNF | Tumor Necrosis Factor |
| TRAIL | Tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| UAE | Ultrasound-assisted extraction |
| UPR | Unfolded protein response |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless related integration site |
| XIAP | X-chromosome-linked inhibitor of apoptosis protein |
| ADPC | Androgen-dependant prostate cancer |
| AIPC | Androgen-independent prostate cancer |
| AP | Activator protein |
| Bcl | B cell lymphoma |
| Bcl-xL | B cell lymphoma-extra large |
| COX | Cyclooxygenase |
| CXCL | Chemokine (C-X-C motif) ligand |
| FOX | Forkhead box protein |
| IL | Interleukin |
| JNK | Jun N-terminal kinase |
| MMP | Matrix metalloproteinase |
| MPA | Medroxyprogesterone acetate |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ODC | Ornithine decarboxylase |
| PECAM | Platelet endothelial cell adhesion molecule |
| PLGA | Poly(D,L-Lactic-co-glycolic acid) |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| TRAIL | Tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| VEGF | Vascular endothelial growth factor |
| XIAP | X-chromosome-linked inhibitor of apoptosis protein |
References
- Rao, A.S.; Hegde, S.; Pacioretty, L.M.; DeBenedetto, J.; Babish, J.G. Nigella sativa and trigonella foenum-graecum supplemented chapatis safely improve HbA1c, body weight, waist circumference, blood lipids, and fatty liver in overweight and diabetic subjects: A twelve-week safety and efficacy study. J. Med. Food 2020, 23, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Trottier, G.; Boström, P.J.; Lawrentschuk, N.; Fleshner, N.E. Nutraceuticals and prostate cancer prevention: A current review. Nat. Rev. Urol. 2010, 7, 21–30. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and functional food as future food: A review. Der Pharm. Lett. 2010, 2, 106–116. [Google Scholar]
- Murphy, R. Extra-virgin olive oil has similar activity to ibuprofen. Nat. Clin. Pract. Rheumatol. 2005, 1, 66. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G. Daily use of extra virgin olive oil with high oleocanthal concentration reduced body weight, waist circumference, alanine transaminase, inflammatory cytokines and hepatic steatosis in subjects with the metabolic syndrome: A 2-month intervention study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Canistro, D.; Chiavaroli, A.; Cicia, D.; Cimino, F.; Curro, D.; Dell’Agli, M.; Ferrante, C.; Giovannelli, L.; Leone, S.; Martinelli, G. The pharmacological basis of the curcumin nutraceutical uses: An update. Pharmadvances 2021, 3, 421–466. [Google Scholar] [CrossRef]
- Vogel, A.; Pelletier, J. Examen chimique de la racine de Curcuma. J. Pharm. 1815, 1, 289–300. [Google Scholar]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Lal, J. Turmeric, curcumin and our life: A review. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 11–17. [Google Scholar]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science & Business Media: Cham, Switzerland, 2007. [Google Scholar]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Lin, C.-L.; Lin, J.-K. Curcumin: A potential cancer chemopreventive agent through suppressing NF-κB signaling. J. Cancer Mol. 2008, 4, 11–16. [Google Scholar]
- Shen, L.; Ji, H.-F. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Synthesis and Anti Diabetic Activity of Curcumin. Int. J. Adv. Res. Ideal Innov. Technol. 2017, 3, 1512–1520. [Google Scholar]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Volak, L.P.; Hanley, M.J.; Masse, G.; Hazarika, S.; Harmatz, J.S.; Badmaev, V.; Majeed, M.; Greenblatt, D.J.; Court, M.H. Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br. J. Clin. Pharmacol. 2013, 75, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Lukita-Atmadja, W.; Ito, Y.; Baker, G.L.; McCuskey, R.S. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock 2002, 17, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design. J. Pharm. Biomed. Anal. 2008, 46, 322–327. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crops Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Osorio-Tobon, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: Process integration and economic evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Liang, H.; Zhang, Q.; Li, Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crops Prod. 2015, 76, 487–493. [Google Scholar] [CrossRef]
- Search Media-Wikimedia Commons. (n.d.). Available online: https://commons.wikimedia.org/w/index.php?search=Curcumin+structure&title=Special:MediaSearch&go=Go&type=image (accessed on 24 April 2022).
- Search Media-Wikimedia Commons. (n.d.). Available online: https://commons.wikimedia.org/w/index.php?search=turmeric&title=Special:MediaSearch&go=Go&type=image (accessed on 24 April 2022).
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H. Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-B pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Garufi, A.; Giorno, E.; Gilardini Montani, M.S.; Pistritto, G.; Crispini, A.; Cirone, M.; D’Orazi, G. p62/SQSTM1/Keap1/NRF2 axis reduces cancer cells death-sensitivity in response to Zn (II)–curcumin complex. Biomolecules 2021, 11, 348. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF- B and P2X7R signaling in PMA-induced macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS induced septic shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Fujisawa, S. Expression of cyclooxygenase-2, nitric oxide synthase 2 and heme oxygenase-1 mRNA induced by bis-eugenol in RAW264. 7 cells and their antioxidant activity determined using the induction period method. In Vivo 2017, 31, 819–831. [Google Scholar]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef]
- Huang, G.; Xu, Z.; Huang, Y.; Duan, X.; Gong, W.; Zhang, Y.; Fan, J.; He, F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J. Clin. Immunol. 2013, 33, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, S.; Cassione, E.B.; De Stefano, L.; Manzo, A. Established rheumatoid arthritis. The pathogenic aspects. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101478. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Kim, M.-O.; Choi, Y.H.; Park, Y.-M.; Kim, G.-Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef]
- Dai, Q.; Di Zhou, L.X.; Song, X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des. Devel. Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, Y.; Zheng, S.; Weng, Z.; Ma, J.; Li, Y.; Xie, X.; Zheng, W. Detention of copper by sulfur nanoparticles inhibits the proliferation of A375 malignant melanoma and MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 1031–1037. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Inhibition of the IL-18 receptor signaling pathway ameliorates disease in a murine model of rheumatoid arthritis. Cells 2019, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phyther. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Fu, M.; Zhou, H.; Li, Y.; Jin, H.; Liu, X. Global, regional, and national burdens of hip osteoarthritis from 1990 to 2019: Estimates from the 2019 Global Burden of Disease Study. Arthritis Res. Ther. 2022, 24, 8. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, E.; Griffin, T.M.; Humphrey, M.B. Innate Immune Responses and Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.; Paul, I.; Rose, R. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet 1972, 299, 519–522. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Favre, J.; Erhart-Hledik, J.C.; Chu, C.R. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann. Biomed. Eng. 2015, 43, 376–387. [Google Scholar] [CrossRef]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [Green Version]
- Felson, D.T.; Lawrence, R.C.; Hochberg, M.C.; McAlindon, T.; Dieppe, P.A.; Minor, M.A.; Blair, S.N.; Berman, B.M.; Fries, J.F.; Weinberger, M. Osteoarthritis: New insights. Part 2: Treatment approaches. Ann. Intern. Med. 2000, 133, 726–737. [Google Scholar] [CrossRef]
- Smalley, W.E.; Ray, W.A.; Daugherty, J.R.; Griffin, M.R. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am. J. Epidemiol. 1995, 141, 539–545. [Google Scholar] [CrossRef]
- Pitt, B.; Pepine, C.; Willerson, J.T. Cyclooxygenase-2 inhibition and cardiovascular events. Circulation 2002, 106, 167–169. [Google Scholar] [CrossRef] [Green Version]
- Hörl, W.H. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals 2010, 3, 2291–2321. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin Prevents Osteoarthritis by Inhibiting the Activation of Inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [Green Version]
- Yabas, M.; Orhan, C.; Er, B.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Bhanuse, P.; Morde, A.A.; Padigaru, M.; et al. A Next Generation Formulation of Curcumin Ameliorates Experimentally Induced Osteoarthritis in Rats via Regulation of Inflammatory Mediators. Front. Immunol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Benderdour, M.; Martel-Pelletier, J.; Pelletier, J.-P.; Kapoor, M.; Zunzunegui, M.-V.; Fahmi, H. Cellular aging, senescence and autophagy processes in osteoarthritis. Curr. Aging Sci. 2015, 8, 147–157. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Nirvanashetty, S.; Parachur, V.A.; Mohanty, N.; Swain, T. A Randomized, Double Blind, Placebo Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Curene® versus Placebo in Reducing Symptoms of Knee OA. Biomed. Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Gharbi, M.; Dierckxsens, Y.; Priem, F.; Marty, M.; Seidel, L.; Albert, A.; Heuse, E.; Bonnet, V.; Castermans, C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis: A randomized trial. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Strier, L.; Kazanov, D.; Elkayam, O.; Lichtenberg, D.; Caspi, D.; Arber, N. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology 2006, 45, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators inspired by nature: A review on curcumin and echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Mezzogori, D.; Rolesi, R.; Eramo, S.L.M.; Paludetti, G.; Troiani, D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: The role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer 2015, 113, 1434–1444. [Google Scholar] [CrossRef] [Green Version]
- Deeb, D.; Jiang, H.; Gao, X.; Divine, G.; Dulchavsky, S.A.; Gautam, S.C. Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J. Exp. Ther. Oncol. 2005, 5, 81–91. [Google Scholar]
- Deeb, D.; Jiang, H.; Gao, X.; Al-Holou, S.; Danyluk, A.L.; Dulchavsky, S.A.; Gautam, S.C. Curcumin [1, 7-bis (4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3, 5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-κB via inhibition of the Prosurvival Akt Signaling Pathway. J. Pharmacol. Exp. Ther. 2007, 321, 616–625. [Google Scholar] [PubMed]
- De Velasco, M.A.; Lu, Y.; Kura, Y.; China, T.; Inoue, Y.; Nakayama, A.; Okada, H.; Horie, S.; Uemura, H.; Ide, H. Chemopreventive effects of nanoparticle curcumin in a mouse model of Pten-deficient prostate cancer. Hum. Cell 2020, 33, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qian, W.; Tao, W.; Zhou, Y.; Xue, B. Delivery of curcumin nanoliposomes using surface modified with CD133 aptamers for prostate cancer. Drug Des. Devel. Ther. 2019, 13, 4021–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor–related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-κB through suppression of IκBα phosphorylation. Mol. Cancer Ther. 2004, 3, 803–812. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Bueso-Ramos, C.; Chatterjee, D.; Pantazis, P.; Aggarwal, B.B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001, 20, 7597–7609. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, Z.; Hu, Z.; Zeng, X.; Li, Y.; Su, Y.; Zhang, C.; Ye, Z. Anti-tumor activity of curcumin against androgen-independent prostate cancer cells via inhibition of NF-κB and AP-1 pathway in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 530–534. [Google Scholar] [CrossRef]
- Hour, T.; Chen, J.; Huang, C.; Guan, J.; Lu, S.; Pu, Y. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21WAF1/CIP1 and C/EBPβ expressions and suppressing NF-κB activation. Prostate 2002, 51, 211–218. [Google Scholar] [CrossRef]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and-2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef] [Green Version]
- Deeb, D.; Xu, Y.X.; Jiang, H.; Gao, X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in lncap prostate cancer cells1. Mol. Cancer Ther. 2003, 2, 95–103. [Google Scholar]
- Andrzejewski, T.; Deeb, D.; Gao, X.; Danyluk, A.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Therapeutic efficacy of curcumin/TRAIL combination regimen for hormone-refractory prostate cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2008, 17, 257–267. [Google Scholar] [CrossRef]
- Gracia, E.; Mancini, A.; Colapietro, A.; Mateo, C.; Gracia, I.; Festuccia, C.; Carmona, M. Impregnation of curcumin into a biodegradable (poly-lactic-co-glycolic acid, PLGA) support, to transfer its well known in vitro effect to an in vivo prostate cancer model. Nutrients 2019, 11, 2312. [Google Scholar] [CrossRef] [Green Version]
- Poma, P.; Labbozzetta, M.; D’Alessandro, N.; Notarbartolo, M. NF-κB is a potential molecular drug target in triple-negative breast cancers. Omi. A J. Integr. Biol. 2017, 21, 225–231. [Google Scholar] [CrossRef]
- RS, P.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Dual drug delivery of curcumin and niclosamide using PLGA nanoparticles for improved therapeutic effect on breast cancer cells. J. Polym. Res. 2020, 27, 133. [Google Scholar]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf. B Biointerfaces 2018, 167, 63–72. [Google Scholar] [CrossRef]
- Prabhuraj, R.S.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Selection of superior targeting ligands using PEGylated PLGA nanoparticles for delivery of curcumin in the treatment of triple-negative breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101722. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S.F.W. NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS ONE 2017, 12, e0169622. [Google Scholar]
- Park, Y.H. The nuclear factor-kappa B pathway and response to treatment in breast cancer. Pharmacogenomics 2017, 18, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Leu, T.-H.; Maa, M.-C. The molecular mechanisms for the antitumorigenic effect of curcumin. Curr. Med. Chem. Agents 2002, 2, 357–370. [Google Scholar]
- Carroll, C.E.; Ellersieck, M.R.; Hyder, S.M. Curcumin inhibits MPA-induced secretion of VEGF from T47-D human breast cancer cells. Menopause 2008, 15, 570–574. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer. Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surfaces B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Cytotoxicity of curcumin silica nanoparticle complexes conjugated with hyaluronic acid on colon cancer cells. Int. J. Biol. Macromol. 2015, 74, 162–170. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Khaniki, M.; Azizian, S.; Mohaghgheghi, M.A.; Sadeghizadeh, M.; Najafi, F. Chemoprevention of azoxymethane-initiated colon cancer in rat by using a novel polymeric nanocarrier–curcumin. Eur. J. Pharmacol. 2012, 689, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Yu, L.; Zhao, L.-Z. Curcumin up regulates T helper 1 cells in patients with colon cancer. Am. J. Transl. Res. 2017, 9, 1866–1875. [Google Scholar] [PubMed]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Lakshmanan, V.-K.; Jayakumar, R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim. Biophys. Acta (BBA) General Subj. 2014, 1840, 2730–2743. [Google Scholar] [CrossRef]
- Anitha, A.; Sreeranganathan, M.; Chennazhi, K.P.; Lakshmanan, V.-K.; Jayakumar, R. In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N, O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2014, 88, 238–251. [Google Scholar] [CrossRef]
- Esmatabadi, M.J.D.; Farhangi, B.; Safari, Z.; Kazerooni, H.; Shirzad, H.; Zolghadr, F.; Sadeghizadeh, M. Dendrosomal curcumin inhibits metastatic potential of human SW480 colon cancer cells through Down-regulation of Claudin1, Zeb1 and Hef1-1 gene expression. Asian Pac. J. Cancer Prev. 2015, 16, 2473–2481. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.H.; Lord, M.S. Curcumin-loading-dependent stability of PEGMEMA-based micelles affects endocytosis and exocytosis in colon carcinoma cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Waghela, B.N.; Sharma, A.; Dhumale, S.; Pandey, S.M.; Pathak, C. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS ONE 2015, 10, e0117526. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2020, 80, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Jin, C.-Y.; Lee, J.-D.; Choi, Y.H.; Ahn, S.-C.; Lee, C.-M.; Jeong, S.-C.; Park, Y.-M.; Kim, G.-Y. Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-α and IL-1β: Suppression of p38, JNK and NF-κB p65 as potential targets. Biol. Pharm. Bull. 2006, 29, 1470–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Senanayake, T.H.; Bohling, A.; Vinogradov, S.V. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: Synthesis, pharmacokinetics, and tumor growth inhibition. Mol. Pharm. 2014, 11, 3112–3122. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Ebeling, M.C.; Khan, S.; Sundram, V.; Chauhan, N.; Gupta, B.K.; Puumala, S.E.; Jaggi, M.; Chauhan, S.C. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol. Cancer Ther. 2013, 12, 1471–1480. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Ahmad, A.; Banerjee, S.; Padhye, S.; Dominiak, K.; Schaffert, J.M.; Wang, Z.; Philip, P.A.; Sarkar, F.H. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010, 70, 3606–3617. [Google Scholar] [CrossRef] [Green Version]
- Bimonte, S.; Barbieri, A.; Palma, G.; Luciano, A.; Rea, D.; Arra, C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res. Int. 2013, 2013, 810423. [Google Scholar] [CrossRef]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Osterman, C.J.D.; Wall, N.R. Curcumin and pancreatic cancer: A research and clinical update. J. Nat. Sci. 2015, 1, e124. [Google Scholar]
- Li, J.; Wang, Y.; Yang, C.; Wang, P.; Oelschlager, D.K.; Zheng, Y.; Tian, D.-A.; Grizzle, W.E.; Buchsbaum, D.J.; Wan, M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Ndinguri, M.W.; Solipuram, R.; Wakamatsu, N.; Hammer, R.P.; Ingram, D.; Hansel, W. [DLys6] luteinizing hormone releasing hormone-curcumin conjugate inhibits pancreatic cancer cell growth in vitro and in vivo. Int. J. Cancer 2011, 129, 1611–1623. [Google Scholar] [CrossRef] [Green Version]
- Sahu, R.P.; Batra, S.; Srivastava, S.K. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer 2009, 100, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef] [Green Version]
- Lev-Ari, S.; Starr, A.; Vexler, A.; Karaush, V.; Loew, V.; Greif, J.; Fenig, E.; Aderka, D.; Ben-Yosef, R. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006, 26, 4423–4430. [Google Scholar]
- Basha, R.; Connelly, S.F.; Sankpal, U.T.; Nagaraju, G.P.; Patel, H.; Vishwanatha, J.K.; Shelake, S.; Tabor-Simecka, L.; Shoji, M.; Simecka, J.W. Small molecule tolfenamic acid and dietary spice curcumin treatment enhances antiproliferative effect in pancreatic cancer cells via suppressing Sp1, disrupting NF-kB translocation to nucleus and cell cycle phase distribution. J. Nutr. Biochem. 2016, 31, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moragoda, L.; Jaszewski, R.; Majumdar, A.P. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001, 21, 873–878. [Google Scholar]
- Geetha, P.; Sivaram, A.J.; Jayakumar, R.; Mohan, C.G. Integration of in silico modeling, prediction by binding energy and experimental approach to study the amorphous chitin nanocarriers for cancer drug delivery. Carbohydr. Polym. 2016, 142, 240–249. [Google Scholar] [CrossRef]
- Qin, H.B.; Wei, L.; Zhang, J.W.; Tang, J.M. Study on functions and mechanism of curcumin in inducing gastric carcinoma BGC apoptosis. Chinese J. Cell. Mol. Immunol. 2011, 27, 1227–1230. [Google Scholar]
- Cai, X.Z.; Huang, W.Y.; Qiao, Y.; Du, S.Y.; Chen, Y.; Chen, D.; Yu, S.; Che, R.C.; Liu, N.; Jiang, Y. Inhibitory effects of curcumin on gastric cancer cells: A proteomic study of molecular targets. Phytomedicine 2013, 20, 495–505. [Google Scholar] [CrossRef]
- Gupta, N.; Aggarwal, N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin—“A technical note”. AAPS PharmSciTech 2008, 9, 810–813. [Google Scholar]
- Arya, P.; Pathak, K. Assessing the viability of microsponges as gastro retentive drug delivery system of curcumin: Optimization and pharmacokinetics. Int. J. Pharm. 2014, 460, 1–12. [Google Scholar] [CrossRef]
- Dhivya, R.; Ranjani, J.; Bowen, P.K.; Rajendhran, J.; Mayandi, J.; Annaraj, J. Biocompatible curcumin loaded PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in human gastric cancer cells. Mater. Sci. Eng. C 2017, 80, 59–68. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Li, N.; Wang, N.; Wu, T.; Qiu, C.; Wang, X.; Jiang, S.; Zhang, Z.; Liu, T.; Wei, C.; Wang, T. Preparation of curcumin-hydroxypropyl-β-cyclodextrin inclusion complex by cosolvency-lyophilization procedure to enhance oral bioavailability of the drug. Drug Dev. Ind. Pharm. 2018, 44, 1966–1974. [Google Scholar] [CrossRef]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cárdenas, A.; Miranda, V.; Robert, P.; Leyton, L. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef]
- Ibrahim, S.; Tagami, T.; Kishi, T.; Ozeki, T. Curcumin marinosomes as promising nano-drug delivery system for lung cancer. Int. J. Pharm. 2018, 540, 40–49. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Kumari, P.; Ray, E.; Ghosh, B.; Biswas, S. Curcumin-loaded chitosan—Cholesterol micelles: Evaluation in monolayers and 3D cancer spheroid model. Nanomedicine 2017, 12, 1435–1453. [Google Scholar] [CrossRef]
- Kim, K.-C.; Baek, S.-H.; Lee, C. Curcumin-induced downregulation of Axl receptor tyrosine kinase inhibits cell proliferation and circumvents chemoresistance in non-small lung cancer cells. Int. J. Oncol. 2015, 47, 2296–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, I.; Wang, W.-S.; Liu, H.-C.; Yang, S.-T.; Tang, N.-Y.; Chung, J.-G. Curcumin alters gene expression-associated DNA damage, cell cycle, cell survival and cell migration and invasion in NCI-H460 human lung cancer cells in vitro. Oncol. Rep. 2015, 34, 1853–1874. [Google Scholar] [CrossRef] [PubMed]
- Sathuvan, M.; Thangam, R.; Gajendiran, M.; Vivek, R.; Balasubramanian, S.; Nagaraj, S.; Gunasekaran, P.; Madhan, B.; Rengasamy, R. κ-Carrageenan: An effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr. Polym. 2017, 160, 184–193. [Google Scholar] [CrossRef]
- Tunc, D.; Dere, E.; Karakas, D.; Cevatemre, B.; Yilmaz, V.T.; Ulukaya, E. Cytotoxic and apoptotic effects of the combination of palladium (II) 5, 5-diethylbarbiturate complex with bis (2-pyridylmethyl) amine and curcumin on non small lung cancer cell lines. Bioorg. Med. Chem. 2017, 25, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Pandey, R.S.; Kush, P.; Kaushik, D.; Jain, U.K.; Madan, J. Inhalable bioresponsive chitosan microspheres of doxorubicin and soluble curcumin augmented drug delivery in lung cancer cells. Int. J. Biol. Macromol. 2017, 98, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, C.; Zhang, J.; Liu, B.; Wei, Z.; Fan, X.; Sui, Z.; Tan, Q. Catanionic lipid nanosystems improve pharmacokinetics and anti-lung cancer activity of curcumin. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-Q.; Xing, L.; Cui, P.-F.; Qiao, J.-B.; He, Y.-J.; Chen, B.-A.; Jin, L.; Jiang, H.-L. Curcumin-coordinated nanoparticles with improved stability for reactive oxygen species-responsive drug delivery in lung cancer therapy. Int. J. Nanomed. 2017, 12, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Paunovic, V.; Ristic, B.; Markovic, Z.; Todorovic-Markovic, B.; Kosic, M.; Prekodravac, J.; Kravic-Stevovic, T.; Martinovic, T.; Micusik, M.; Spitalsky, Z. c-Jun N-terminal kinase-dependent apoptotic photocytotoxicity of solvent exchange-prepared curcumin nanoparticles. Biomed. Microdevices 2016, 18, 37. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Wang, L.; Zhou, X.; Chan, J.Y.-W.; Li, J.; Cui, G.; Lee, S.M.-Y. Synergistic effect of fenretinide and curcumin for treatment of non-small cell lung cancer. Cancer Biol. Ther. 2016, 17, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadehdehkordi, M.; Adelinik, A.; Tashakor, A. Dual effect of curcumin targets reactive oxygen species, adenosine triphosphate contents and intermediate steps of mitochondria-mediated apoptosis in lung cancer cell lines. Eur. J. Pharmacol. 2015, 769, 203–210. [Google Scholar] [CrossRef]
- Srivastava, S.; Mohammad, S.; Pant, A.B.; Mishra, P.R.; Pandey, G.; Gupta, S.; Farooqui, S. Co-delivery of 5-fluorouracil and curcumin nanohybrid formulations for improved chemotherapy against oral squamous cell carcinoma. J. Maxillofac. Oral Surg. 2018, 17, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Kundu, A.; Pradhan, D.; Jyoti, B.; Chokotiya, H.; Parashar, P. A Comparative Study to Evaluate the Efficacy of Curcumin Lozenges (TurmNova®) and Intralesional Corticosteroids with Hyaluronidase in Management of Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 2021, 22, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Enhancement of phototoxicity of curcumin in human oral cancer cells using silica nanoparticles as delivery vehicle. Lasers Med. Sci. 2014, 29, 645–652. [Google Scholar] [CrossRef]
- De Souza Ferreira, S.B.; Slowik, K.M.; de Castro Hoshino, L.V.; Baesso, M.L.; Murdoch, C.; Colley, H.E.; Bruschi, M.L. Mucoadhesive emulgel systems containing curcumin for oral squamous cell carcinoma treatment: From pre-formulation to cytotoxicity in tissue-engineering oral mucosa. Eur. J. Pharm. Sci. 2020, 151, 105372. [Google Scholar]
- Ardito, F.; Perrone, D.; Giuliani, M.; Testa, N.F.; Muzio, L.L. Effects of curcumin on squamous cell carcinoma of tongue: An in vitro study. Curr. Top. Med. Chem. 2018, 18, 233–243. [Google Scholar] [CrossRef]
- Ba, P.; Xu, M.; Yu, M.; Li, L.; Duan, X.; Lv, S.; Fu, G.; Yang, J.; Yang, P.; Yang, C. Curcumin suppresses the proliferation and tumorigenicity of Cal27 by modulating cancer associated fibroblasts of TSCC. Oral Dis. 2020, 26, 1375–1383. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective skin cancer treatment by topical co-delivery of curcumin and STAT3 siRNA using cationic liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Peram, M.R.; Jalalpure, S.; Kumbar, V.; Patil, S.; Joshi, S.; Bhat, K.; Diwan, P. Factorial design based curcumin ethosomal nanocarriers for the skin cancer delivery: In vitro evaluation. J. Liposome Res. 2019, 29, 291–311. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Huang, F.; Liu, B.; Xie, Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J. Lipid Res. 2016, 57, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.-M.; Kang, J.-H.; Kawada, T.; Yoo, H.; Sung, M.-K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic properties of curcumin II: Evidence from in vivo studies. Nutrients 2019, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Suresh Babu, P.; Srinivasan, K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol. Cell. Biochem. 1998, 181, 87–96. [Google Scholar] [CrossRef]
- Hussain, A. Hypoglycemic, hypolipidemic and antioxidant properties of combination ofCurcumin from Curcuma longa, Linn, and partially purified product fromAbroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. 2002, 17, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Murugan, P.; Pari, L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamideinduced diabetic rats. Life Sci. 2006, 79, 1720–1728. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Satyanarayana, A.; Balakrishna, N.; Kumar, P.U.; Reddy, G.B. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med. Sci. Monit. 2007, 13, BR286–BR292. [Google Scholar]
- Tikoo, K.; Meena, R.L.; Kabra, D.G.; Gaikwad, A.B. Change in post translational modifications of histone H3, heat shock protein 27 and MAP kinase p38 expression by curcumin in streptozotocin induced type I diabetic nephropathy. Br. J. Pharmacol. 2008, 153, 1225–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanitkar, M.; Gokhale, K.; Galande, S.; Bhonde, R.R. Novel role of curcumin in the prevention of cytokine induced islet death in vitro and diabetogenesis in vivo. Br. J. Pharmacol. 2008, 155, 702–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pari, L.; Karthikesan, K.; Menon, V.P. Comparative and combined effect of chlorogenic acid and tetrahydrocurcumin on antioxidant disparities in chemical induced experimental diabetes. Mol. Cell. Biochem. 2010, 341, 109–117. [Google Scholar] [CrossRef]
- El-Azab, M.F.; Attia, F.M.; El-Mowafy, A.M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Eur. J. Pharmacol. 2011, 658, 41–48. [Google Scholar] [CrossRef]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002, 57, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Razzaghi-Asl, N.; Tikare, S.N.; Di Santo, R.; Costi, R.; Messore, A.; Pescatori, L.; Crucitti, G.C.; Jargar, J.G.; Dhundasi, S.A. Hypoglycemic activity of curcumin synthetic analogues in alloxan-induced diabetic rats. J. Enzyme Inhib. Med. Chem. 2016, 31, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.S.; Gehr, T.W.B.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF- B activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Y.; Luo, M.; Wu, H.; Kong, L.; Xin, Y.; Cui, W.; Zhao, Y.; Wang, J.; Liang, G. Novel curcumin analog C66 prevents diabetic nephropathy via JNK pathway with the involvement of p300/CBP-mediated histone acetylation. Biochim. Biophys. Acta (BBA) Molecular Basis Dis. 2015, 1852, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Domínguez, B.; Aparicio-Trejo, O.E.; García-Arroyo, F.E.; León-Contreras, J.C.; Tapia, E.; Molina-Jijón, E.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rojas, S.H.; Lira-León, A.; Aparicio-Trejo, O.E.; Reyes-Fermín, L.M.; Pedraza-Chaverri, J. Role of autophagy on heavy metal-induced renal damage and the protective effects of curcumin in autophagy and kidney preservation. Medicina 2019, 55, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease (review). Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Khopde, S.M.; Priyadarsini, K.I.; Venkatesan, P.; Rao, M.N.A. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys. Chem. 1999, 80, 85–91. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is A Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. Theochem 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Faten, R.A.; Ibrahim, A.E.; Khaled, A.E. Protective and modulatory effects of Curcumin and L-Carnitine against Methotrexate-induced Oxidative stress in albino rats. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 744–754. [Google Scholar]
- Balamurugan, A.N.; Akhov, L.; Selvaraj, G.; Pugazhenthi, S. Induction of antioxidant enzymes by curcumin and its analogues in human islets: Implications in transplantation. Pancreas 2009, 38, 454–460. [Google Scholar] [CrossRef]
- Meghana, K.; Sanjeev, G.; Ramesh, B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577, 183–191. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, F.; Barreto, G.; Majeed, M.; Sahebkar, A. Modulatory effects of curcumin on heat shock proteins in cancer: A promising therapeutic approach. BioFactors 2019, 45, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Sashidhar, R.B. Metabolic intervention of aflatoxin B1 toxicity by curcumin. J. Ethnopharmacol. 2010, 127, 641–644. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: A review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin derivatives verify the essentiality of ROS upregulation in tumor suppression. Molecules 2019, 24, 4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J.Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Araveti, P.B.; Srivastava, A. Curcumin induced oxidative stress causes autophagy and apoptosis in bovine leucocytes transformed by Theileria annulata. Cell Death Discov. 2019, 5, 100. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Contreras, J.L.; Smyth, C.A.; Bilbao, G.; Eckstein, C.; Young, C.J.; Thompson, J.A.; Curiel, D.T.; Eckhoff, D.E. Coupling endoplasmic reticulum stress to cell death program in isolated human pancreatic islets: Effects of gene transfer of Bcl 2. Transpl. Int. 2003, 16, 537–542. [Google Scholar] [CrossRef]
- Ye, M.; Qiu, H.; Cao, Y.; Zhang, M.; Mi, Y.; Yu, J.; Wang, C. Curcumin improves palmitate-induced insulin resistance in human umbilical vein endothelial cells by maintaining proteostasis in endoplasmic reticulum. Front. Pharmacol. 2017, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 2012, 81, 767–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, K.; Sil, P.C. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol. Appl. Pharmacol. 2015, 282, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Chang, C.L.; Yu, C.S.; Chen, P.Y.; Yang, J.S.; Ji, B.C.; Lin, T.P.; Chiu, C.F.; Yeh, S.P.; Huang, Y.P. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI 3 cells is mediated via endoplasmic reticulum stress and mitochondria dependent pathways. Environ. Toxicol. 2013, 28, 255–266. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef]
- Lin, S.-S.; Huang, H.-P.; Yang, J.-S.; Wu, J.-Y.; Hsai, T.-C.; Lin, C.-C.; Lin, C.-W.; Kuo, C.-L.; Wood, W.G.; Chung, J.-G. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade-and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90. [Google Scholar] [CrossRef]
- Pae, H.-O.; Jeong, S.-O.; Jeong, G.-S.; Kim, K.M.; Kim, H.S.; Kim, S.-A.; Kim, Y.-C.; Kang, S.-D.; Kim, B.-N.; Chung, H.-T. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem. Biophys. Res. Commun. 2007, 353, 1040–1045. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Booijink, C.C.G.M.; El-Aidy, S.; Rajilić-Stojanović, M.; Heilig, H.G.H.J.; Troost, F.J.; Smidt, H.; Kleerebezem, M.; De Vos, W.M.; Zoetendal, E.G. High temporal and inter-individual variation detected in the human ileal microbiota. Environ. Microbiol. 2010, 12, 3213–3227. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Raes, J.; Van Den Bogert, B.; Arumugam, M.; Booijink, C.C.G.M.; Troost, F.J.; Bork, P.; Wels, M.; De Vos, W.M.; Kleerebezem, M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta (BBA) General Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, R.; Kumar, T.R.S.; Karunagaran, D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003, 538, 19–24. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [Green Version]
- Khoury, T.; Rmeileh, A.A.; Yosha, L.; Benson, A.A.; Daher, S.; Mizrahi, M. Drug induced liver injury: Review with a focus on genetic factors, tissue diagnosis, and treatment options. J. Clin. Transl. Hepatol. 2015, 3, 99–108. [Google Scholar] [PubMed]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1 and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef] [Green Version]
- Vecchi Brumatti, L.; Marcuzzi, A.; Tricarico, P.M.; Zanin, V.; Girardelli, M.; Bianco, A.M. Curcumin and inflammatory bowel disease: Potential and limits of innovative treatments. Molecules 2014, 19, 21127–21153. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF- B activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Martelli, L.; Ragazzi, E.; Di Mario, F.; Martelli, M.; Castagliuolo, I.; Dal Maschio, M.; Palù, G.; Maschietto, M.; Scorzeto, M.; Vassanelli, S. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol. Motil. 2007, 19, 668–674. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story So Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Bais, H.P. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Ammayappan, L.; Moses, J.J. Study of antimicrobial activity of aloevera, chitosan, and curcumin on cotton, wool, and rabbit hair. Fibers Polym. 2009, 10, 161–166. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Chen, D.Y.; Shien, J.H.; Tiley, L.; Chiou, S.S.; Wang, S.Y.; Chang, T.J.; Lee, Y.J.; Chan, K.W.; Hsu, W.L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Ingolfsson, H.I.; Koeppe, R.E.; Andersen, O.S. Curcumin is a Modulator of Bilayer Material Properties. Biochemistry 2007, 46, 10384–10391. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.P.; Bankwitz, D.; Steinmann, J.; Ott, M. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Yoo, H.S.; Kim, J.C.; Park, C.S.; Choi, M.S.; Kim, M.; Choi, H.; Min, J.S.; Kim, Y.S.; Yoon, S.W.; et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009, 124, 189–196. [Google Scholar] [CrossRef]
- Narayanan, A.; Kehn-Hall, K.; Senina, S.; Lundberg, L.; Van Duyne, R.; Guendel, I.; Das, R.; Baer, A.; Bethel, L.; Turell, M.; et al. Curcumin inhibits rift valley fever virus replication in human cells. J. Biol. Chem. 2012, 287, 33198–33214. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; de Montellano, P.R.O. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef]
- Ali, A.; Banerjea, A.C. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016, 6, 27539. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kulkarni, A.A.; Lin, X.; McLean, C.; Ammosova, T.; Ivanov, A.; Hipolito, M.; Nekhai, S.; Nwulia, E. Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des. Devel. Ther. 2015, 9, 5051–5060. [Google Scholar]
- Devadas, K.; Hewlett, I.K.; Dhawan, S. Lipopolysaccharide suppresses HIV 1 replication in human monocytes by protein kinase C dependent heme oxygenase 1 induction. J. Leukoc. Biol. 2010, 87, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.L.C.; Abreu, M.P.; Costa, C.B.; Leda, P.O.; Behrens, M.D.; Dos Santos, E.P. Curcumin and Its Analogs as a Therapeutic Strategy in Infections Caused by RNA Genome Viruses. Food Environ. Virol. 2022, 14, 120–137. [Google Scholar] [CrossRef]
- Avasarala, S.; Zhang, F.; Liu, G.; Wang, R.; London, S.D.; London, L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS ONE 2013, 8, e57285. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Cai, Y.; Yu, Y. Effects of a novel curcumin derivative on the functions of kidney in streptozotocin-induced type 2 diabetic rats. Inflammopharmacology 2018, 26, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Liu, Y.; Li, C.; Wang, X.; Zhu, R.; Liu, C.; Liu, H.; Wang, L.; Ma, R.; Fu, M. Recent advances of curcumin in the prevention and treatment of renal fibrosis. Biomed Res. Int. 2017, 2017, 2418671. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xu, J.; Guo, X.; Huang, M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin. Exp. Pharmacol. Physiol. 2018, 45, 84–93. [Google Scholar] [CrossRef]
- Richart, S.M.; Li, Y.-L.; Mizushina, Y.; Chang, Y.-Y.; Chung, T.-Y.; Chen, G.-H.; Tzen, J.T.-C.; Shia, K.-S.; Hsu, W.-L. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J. Food Drug Anal. 2018, 26, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Yan, Y.; Liao, S.; Li, Y.; Ye, Y.; Liu, N.; Zhao, F.; Xu, P. 3D-quantitative structure—Activity relationship and antiviral effects of curcumin derivatives as potent inhibitors of influenza H1N1 neuraminidase. Arch. Pharm. Res. 2020, 43, 489–502. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antiviral Res. 2019, 162, 71–78. [Google Scholar] [CrossRef]
- Padilla, S.L.; Rodríguez, A.; Gonzales, M.M.; Gallego, G.J.C.; Castaño, O.J.C. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch. Virol. 2014, 159, 573–579. [Google Scholar] [CrossRef]
- Huang, H.-I.; Chio, C.-C.; Lin, J.-Y. Inhibition of EV71 by curcumin in intestinal epithelial cells. PLoS ONE 2018, 13, e0191617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Chang, L.; Chu, H.W.; Lin, H.J.; Chang, P.C.; Wang, R.Y.L.; Unnikrishnan, B.; Mao, J.Y.; Chen, S.Y.; Huang, C.C. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef]
- Martins, C.V.B.; Da Silva, D.L.; Neres, A.T.M.; Magalhães, T.F.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; De Fátima, Â.; De Resende, M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar] [CrossRef]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, J.H.; Rinaldi, M.G.; Pfaller, M.A. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 1995, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.C.; Marr, K.A.; Bowden, R.A.; Apisariyakul, A.; Vanittanakom, N.; Buddhasukh, D.; Rex, J.H.; Rinaldi, M.G.; Pfaller, M.A.; Dovigo, L.N.; et al. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Photochem. Photobiol. 1995, 49, 382–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 742, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Negi, A.S.; Prasad, R. Synergistic anticandidal activity of pure polyphenol curcumin i in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010, 10, 570–578. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. An antifungal mechanism of curcumin lies in membrane-targeted action within Candida albicans. IUBMB Life 2014, 66, 780–785. [Google Scholar] [CrossRef]
- Khan, N.; Shreaz, S.; Bhatia, R.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012, 83, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomes, A.S.; Curvelo, J.A.R.; Soares, R.M.A.; Ferreira-Pereira, A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med. Mycol. 2012, 50, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Brasch, J.; Freitag-Wolf, S.; Beck-Jendroschek, V.; Huber, M. Inhibition of dermatophytes by photodynamic treatment with curcumin. Med. Mycol. 2017, 55, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol. B Biol. 2017, 173, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Shinobu-Mesquita, C.S.; Bertoni, T.A.; Guilhermetti, E.; Svidzinski, T.I.E. Antifungal activity of the extract of Curcuma zedoaria against yeasts of the genus Candida isolated from the oral cavity of patients infected with the human immunodeficiency virus. Rev. Bras. Farmacogn. 2011, 21, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.D.S.; Amorim, Y.M.; da Silva, E.L.; Silva, S.C.; Santos, A.J.A.; Peixoto, F.N.; Neves Pires, L.M.; Sakamoto, R.Y.; Horta Pinto, F.D.C.; Scarpa, M.V.C.; et al. Formulation, stability study and preclinical evaluation of a vaginal cream containing curcumin in a rat model of vulvovaginal candidiasis. Mycoses 2018, 61, 723–730. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef]
- Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar]
- Song, J.; Choi, B.; Jin, E.-J.; Yoon, Y.; Choi, K.-H. Curcumin suppresses Streptococcus mutans adherence to human tooth surfaces and extracellular matrix proteins. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Wareham, D.W. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014, 14, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, K.M.; Iranshahi, M.; Yazdi, M.C.; Shahverdi, A.R. The combination effect of curcumin with different antibiotics against Staphylococcus aureus. Int. J. Green Pharm 2009, 3, 141. [Google Scholar]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Burgos Morón, E.; Calderón Montaño, J.M.; Salvador, J.; Robles, A.; López Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Kurien, B.T.; Dillon, S.P.; Dorri, Y.; D’souza, A.; Scofield, R.H. Curcumin does not bind or intercalate into DNA and a note on the grey side of curcumin. Int. J. Cancer 2011, 128, 242–245. [Google Scholar] [CrossRef]
- Danafar, H.; Salehiabar, M.; Barsbay, M.; Rahimi, H.; Ghaffarlou, M.; Arbabi Zaboli, K.; Faghfoori, M.H.; Kaboli, S.; Nosrati, H.; Faghfoori, Z. Curcumin delivery by modified biosourced carbon-based nanoparticles. Nanomedicine 2022, 17, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.K.; Koushki, K.; Sathyapalan, T.; Majeed, M.; Sahebkar, A. PLGA-Based Curcumin Delivery System: An Interesting Therapeutic Approach in the Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2022, 20, 309–323. [Google Scholar] [CrossRef]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 49. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, P.; Yoo, S.W.; Ko, Y.T. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res. Int. 2016, 84, 113–119. [Google Scholar] [CrossRef]
- Sarkar, H.; Li, F.; Wang, Y.; Padhye, Z.S. Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Curr. Pharm. Des. 2010, 16, 1801–1812. [Google Scholar] [CrossRef]
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. 2009, 14, 226–246. [Google Scholar]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Banerjee, Y.; Taranikanti, V.; Bayoumi, R. Triglyceride-mediated pathways and coronary heart disease. Lancet 2010, 376, 956. [Google Scholar] [CrossRef]



| Method Name | Yield | Advantage(s) | Drawback(s) | Reference |
|---|---|---|---|---|
| Microwave-assisted extraction (MAE) | The yield obtained using MAE is 4.98%. The Curcuma longa plant was soaked in methanol and extracted using acetone via a dual heating mechanism under microwave energy. | Safe and cost-effective method. Retains the biological activity of the extracted compounds. | Time-consuming, as the vessel needs to be slowly cooled to avoid the loss of volatile components. Involves an initial high cost for setting up the equipment on a large scale. | [28] |
| Enzyme-assisted extraction (EAE) | The yield obtained using EAE is 5.73%. Turmeric was pretreated using alpha-amylase and amyloglucosidase enzymes. Extraction was performed using N,N- dipropyl ammonium N,N’-dipropylcarbamate. | Mild reaction conditions are cost-effective, eco-friendly and feasible. | Low purity of the final product. | [29] |
| Supercritical fluid extraction (SFE) | The yield obtained using SFE is 4.3%. A two-step extraction was performed using SFE, followed by pressurized liquid extraction using ethanol as solvent. | Short extraction time and mild operating temperature. The manufacturing costs are lower than those of conventional methods. | High technical complexity, and many operational parameters need to be optimized before the initiation of the extraction process. | [30] |
| Ultrasound-assisted extraction (UAE) | The yield obtained using UAE is 1.03%. Optimal conditions were a 60% amplitude and a 3/1 (s/s) pulsed interval. Ethanol was the extraction solvent. | Greater solvent penetration into the samples increases the contact surface area, which increases efficiency. | Ultrasound can lead to the degradation of the final purified product. | [31] |
| Ionic liquid-based extraction (ILE) | The yield obtained using ILE is 6.18%. The ionic liquid used for extraction was an anionic [Omin][Br-] aqueous solution. | Eco-friendly and increases extraction efficiency. | High cost involved in the preparation and use. | [32] |
| Cancer Type | Curcumin Conc. | Signaling Molecules Up/Downregulated | Overview | Delivery Modes | Ref. |
|---|---|---|---|---|---|
| Prostate cancer | 10–100 μM | Downregulates NF-κB, AP-1, Cyclin D1, CXCL-1 and CXCL-2, Bcl-2, Bcl-xL and XIAP. | -Curcumin is a potent inhibitor of NF-κB in both ADPC and AIPC cells, thereby preventing cell proliferation and inducing apoptosis. -Curcumin restores the response of AIPC cells to anti-androgen treatment. -Prevents metastasis in AIPC cells. | -Free curcumin or in combination with chemotherapeutic agents such as TRAIL. -Curcumin in poly(lactic-co-glycolic) acid. -Using the nanoparticle formulation of curcumin. -Curcumin-loaded liposomes. | [84,85,86,87,88,89,90,91,92,93,94,95] |
| Breast cancer | 10–40 μM | Downregulates Bcl-2, CXCL-1, CXCL-2, MMP-9, urokinase plasminogen activator, intercellular adhesion molecule 1 and chemokine receptor 4, PECAM-1, Cyclin D1 and p65. | -Curcumin can suppress ODC activity and inhibit cell proliferation. -Inhibits the MPA-induced secretion of pro-angiogenic factors such as VEGF. | -Hyaluronic acid-modified mesoporous silica nanoparticles. -Chitosan nanoparticles. -Zinc oxide nanoparticles. -In combination with niclosamide using PLGA nanoparticles. -PEGylated PLGA nanoparticles -Nanovesicles. | [21,96,97,98,99,100,101,102,103,104,105,106,107] |
| Colon cancer | 10–50 µM | Downregulates TNFα, JNK activation, miR-21 and COX-2. | -Curcumin inhibits the activation of the TLR4/MyD88/NF-κB signaling axis. -Reduces IκB kinase activity and inhibits the degradation of IkBα. -Inhibits the production of TNF-α, IL-6 and IL-12. -Inhibits Foxp3 expression and enhanced interferon-gamma secretion in regulatory T cells. | -Curcumin in silica nanoparticle. -Polymeric nanocarrier. -Curcumin-loaded thiolated chitosan nanoparticle. -Dendrosomal carrier. -Curcumin-loaded micelles. -Curcumin–PLGA nanoparticles. | [108,109,110,111,112,113,114,115,116,117,118,119] |
| Pancreatic | 10–50 µM | Downregulates EGFR, COX-2, NF-κb, AKT and Prostaglandin E2. | -Inhibited cell survival and enhanced apoptosis in pancreatic adenocarcinoma cell lines. -Suppressed tumor growth by inhibiting the NF-KB pathway. -Induced apoptosis via ATM/Chk1. -Anti-proliferative activity by suppressing Sp1 and disrupting NF-κB translocation to the nucleus. | -Curcumin analogues PEGylated Curcumin, [Dlys6]-LHRH and its analog called L49H37. -CDF and PEGylated curcumin. -Liposomal Curcumin. -Curcumin analogues with the hydroxyl group. -Magnetic particles that were used to encapsulate curcumin. -Ester-mediated conjugations of curcumin to cholesteryl-hyaluronic acid nanogel. | [120,121,122,123,124,125,126,127,128,129,130,131] |
| Gastric | 10–100 µM | Downregulates the Akt pathway, BCL-2, COX-2 and cyclin D1. | -Induced apoptosis by activating caspase-3,PARP; reduction in Bcl-XL levels. -Curcumin also activates the Fas pathway by stimulating the activity of caspase-8. -Activated Bax protein expression and inhibited the Bcl-2 protein -Suppressed the transition of the cancer cells from the G(1) to S phase. | -Cyclodextrin complexes with curcumin. -Nanoparticles such as polymer-encapsulated ZnO nanoparticles. -Microsponges using polymers such as ethyl cellulose and polyvinyl alcohol. -Curcumin-loaded nanoemulsion. -Cationic polysaccharides such as chitosan. | [132,133,134,135,136,137,138,139,140,141,142] |
| Lung | 5–50 µM | Downregulates the Akt pathway, BRCA pathway, Beta-catenin signaling and MMP-2 and upregulates caspase-3, Bax and p53. | Induces apoptotic cell death by activating caspase-7 and 3. -Enhances PARP cleavage and stimulates ER stress. -Enhances ROS production to cause apoptosis. -Increases the sensitivity of cancer cells to chemotherapy. -Induces DNA damage and prevents the migration of cancer cells. | -Lipid-based liposome. -Polymeric carrier and micelle. -Chitosan microsphere. -Polymeric and lipid nanoparticle. -Nanocrystal. | [143,144,145,146,147,148,149,150,151,152,153,154] |
| Oral | 10–100 µM | -Prevents cell proliferation and promotes apoptosis. | -Curcumin reduces the migration and progression of TSCC cells, promotes apoptosis and inhibits tumorigenesis. -Suppresses the CAF (cancer-associated fibroblast)-mediated proliferation and tumorigenicity of Cal27 by inhibiting TSCC CAFs. | -Nanohybrid formulation. -Lozenges. -Silica nanoparticles. -Mucoadhesive nanogel system. | [155,156,157,158,159,160] |
| Skin | 10–50µM | -Shows effective anti-proliferative activity. | -Antiproliferative effect, as they effectively inhibit the clonogenic ability in melanoma cells. | -Cationic liposomes. -Ethosomal nanocarriers. | [161,162] |
| Curcumin Doses | Effect on Gut Microbiota | Molecular Mechanisms | Model | Ref. |
|---|---|---|---|---|
| Curcumin at a low dose (1 g/day) | Curcumin shifted the structure of gut microbiota | Curcumin enervated the Western diet-induced development of atherosclerosis and type 2 diabetes mellitus | Sprague Dawley rats | [225] |
| 100 mg/kg/day | Lowers the increasing abundance of the genera Anaerotruncus and Helicobacter in the gut microbiota | Decreases the estrogen level, resulting in an increase in body weight | Wistar rats | [226] |
| 100 mg/kg/day | Curcumin affected the presence of Prevotellaceae, Bacteroidacea, and Rikenellaceae in gut microbiota | Curcumin possesses anticancer activity in vitro and in preclinical animal models via the activation of caspases 3, 8 and 9 in the colon cancer cell lines | Fecal sample | [227] |
| 8000 mg per day | Increase in Lactobacillus and decrease in Coriobacterales | Induction of apoptosis through the COX-2 and non-COX-2 pathways. It targets cancer stem cells (CSC) through direct or indirect influences on the CSC self-renewal pathways. | Colon cancer cell lines, SW480 and SW62 | [228] |
| 0.2% (w/w) nanoparticles of curcumin | Increase in butyrate-producing bacteria and the fecal butyrate level | Mucosal mRNA expression of inflammatory mediators and the activation of NF-κB in colonic epithelial cells were suppressed by curcumin nanoparticles | BALB/c mice | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12, 639. https://doi.org/10.3390/metabo12070639
Sivani BM, Azzeh M, Patnaik R, Pantea Stoian A, Rizzo M, Banerjee Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites. 2022; 12(7):639. https://doi.org/10.3390/metabo12070639
Chicago/Turabian StyleSivani, Bala Mohan, Mahmoud Azzeh, Rajashree Patnaik, Anca Pantea Stoian, Manfredi Rizzo, and Yajnavalka Banerjee. 2022. "Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions" Metabolites 12, no. 7: 639. https://doi.org/10.3390/metabo12070639
APA StyleSivani, B. M., Azzeh, M., Patnaik, R., Pantea Stoian, A., Rizzo, M., & Banerjee, Y. (2022). Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites, 12(7), 639. https://doi.org/10.3390/metabo12070639