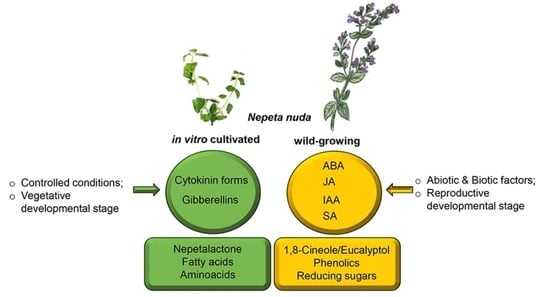
Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. GC-MS Analysis of Volatile Compounds
2.3. GC-MS Analysis of Primary and Secondary Metabolites
2.3.1. Analysis of Amino Acids, Sugars, and Organic Acids
2.3.2. Analysis of Phenolic Acids
2.3.3. Analysis of Fatty Acids, Sterols, and Hydrocarbons
2.4. Anti-Inflammatory Activity
2.5. Anthocyanins and Reducing Sugars
2.6. Plant Hormone Profiling
2.7. Statistics
3. Results
3.1. Volatiles
3.2. Correlation between N. nuda Phytochemicals and Biological Activities
3.3. Compounds in Polar and Non-Polar Fractions
3.4. Composition of Phytohormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Mi, H.M. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kilic, O.; Hayta, S.; Bagci, E. Chemical composition of essential oil of Nepeta nuda L. subsp. nuda (Lamiaceae) from Turkey. Asian J. Chem. 2011, 23, 2788–2790. [Google Scholar]
- Alekseeva, M.; Rusanova, M.; Rusanov, K.; Atanassov, I. A set of highly polymorphic microsatellite markers for genetic diversity studies in the genus Origanum. Plants 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Lončar, B.; Pezo, M.; Stanković Jeremić, J.; Cvetković, M.; Rat, M.; Pezo, L. Volatile compounds of Nepeta nuda L. from Rtanj Mountain (Serbia). Horticulturae 2022, 8, 85. [Google Scholar] [CrossRef]
- Aničić, N.; Matekalo, D.; Skorić, M.; Živković, J.N.; Petrović, L.; Dragićević, M.; Dmitrović, S.; Mišić, D. Alterations in nepetalactone metabolism during polyethylene glycol (PEG)-induced dehydration stress in two Nepeta species. Phytochemistry 2020, 174, 112340. [Google Scholar] [CrossRef]
- Kofidis, G.; Bosabalidis, A.M. Effects of altitude and season on glandular hairs and leaf structural traits of Nepeta nuda L. Bot. Stud. 2008, 49, 363–372. [Google Scholar]
- Eisner, T. Catnip: Its raison d’être. Science 1964, 146, 1318–1320. [Google Scholar] [CrossRef]
- Bozek, M. Pollen efficiency and foraging by insect pollinators in three catnip (Nepeta L.) species. J. Apic. Sci. 2003, 47, 19–24. [Google Scholar]
- Uenoyama, R.; Miyazaki, T.; Hurst, J.L.; Beynon, R.J.; Adachi, M.; Murooka, T.; Onoda, I.; Miyazawa, Y.; Katayama, R.; Yamashita, T.; et al. The characteristic response of domestic cats to plant iridoids allows them to gain chemical defense against mosquitoes. Sci. Adv. 2021, 7, eabd9135. [Google Scholar] [CrossRef]
- Petrova, D.; Gašić, U.; Yocheva, L.; Hinkov, A.; Yordanova, Z.; Chaneva, G.; Mantovska, D.; Paunov, M.; Ivanova, L.; Rogova, M.; et al. Catmint (Nepeta nuda L.) phylogenetics and metabolic responses in variable growth conditions. Front. Plant Sci. 2022, 13, 866777. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mantovska, D.I.; Zhiponova, M.K.; Georgiev, M.I.; Alipieva, K.; Tsacheva, I.; Simova, S.; Yordanova, Z.P. Biological Activity and NMR-Fingerprinting of Balkan endemic species Stachys thracica Davidov. Metabolites 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Lindoo, S.J.; Caldwell, M.M. Ultraviolet-B radiation-induced inhibition of leaf expansion and promotion of anthocyanin production: Lack of involvement of the low irradiance phytochrome system. Plant Physiol. 1978, 61, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Plummer, D.T. An introduction to practical biochemistry. In Biochemical Education, 3rd ed.; McGraw-Hill College: London, UK, 1988; Volume 16, pp. 98–100. [Google Scholar]
- Prerostova, S.; Dobrev, P.I.; Knirsch, V.; Jarosova, J.; Gaudinova, A.; Zupkova, B.; Prášil, I.T.; Janda, T.; Brzobohatý, B.; Skalák, J.; et al. Light quality and intensity modulate cold acclimation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2736. [Google Scholar] [CrossRef]
- Mishev, K.; Dobrev, P.I.; Lacek, J.; Filepová, R.; Yuperlieva-Mateeva, B.; Kostadinova, A.; Hristeva, T. Hormonomic changes driving the negative impact of broomrape on plant host interactions with arbuscular mycorrhizal fungi. Int. J. Mol. Sci. 2021, 22, 13677. [Google Scholar] [CrossRef]
- Principal Components Analysis. Available online: https://search.r-project.org/R/refmans/stats/html/prcomp.html (accessed on 28 September 2023).
- Changes in R 4.3.1. Available online: https://cran.r-project.org/bin/windows/base/NEWS.R-4.3.1.html (accessed on 28 September 2023).
- Ggbiplot. Available online: https://github.com/vqv/ggbiplot (accessed on 28 September 2023).
- Package Stats Version 4.3.1. Correlation, Variance and Covariance (Matrices). Available online: https://search.r-project.org/R/refmans/stats/html/cor.html (accessed on 28 September 2023).
- Tsacheva, I.; Rostan, J.; Iossifova, T.; Vogler, B.; Odjakova, M.; Navas, H.; Kostova, I.; Kojouharova, M.; Kraus, W. Complement inhibiting properties of dragon’s blood from Croton draco. Z. Für Naturforschung C 2004, 59, 528–532. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Kapchina-Toteva, V.; Dimitrova, M.A.; Stefanova, M.; Koleva, D.; Kostov, K.; Yordanova, Z.P.; Stefanov, D.; Zhiponova, M.K. Adaptive changes in photosynthetic performance and secondary metabolites during white dead nettle micropropagation. J. Plant Physiol. 2014, 171, 1344–1353. [Google Scholar] [CrossRef]
- Shan, X.; Yan, J.; Xie, D. Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 2012, 15, 84–91. [Google Scholar] [CrossRef]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.; Genschik, P. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez-Y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Jordi, W.; Kuiper, D. Acclimation of plants to light gradients in leaf canopies: Evidence for a possible role for cytokinins transported in the transpiration stream. J. Exp. Bot. 2001, 52, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Friends in arms: Flavonoids and the auxin/cytokinin balance in terrestrialization. Plants 2023, 12, 517. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.; Babar, M.D. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R.; Ragazinskiene, O. Antioxidant activities and phenolic composition of extracts from Nepeta plant species. In 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production, FOODBALT-2011—Conference Proceedings; Latvia University of Agriculture: Jelgava, Latvia, 2011; pp. 79–83. [Google Scholar]
- Aničić, N.; Gašić, U.; Lu, F.; Ćirić, A.; Ivanov, M.; Jevtić, B.; Dimitrijević, M.; Anđelković, B.; Skorić, M.; Nestorović Živković, J.; et al. Antimicrobial and immunomodulating activities of two endemic Nepeta species and their major iridoids isolated from natural sources. Pharmaceuticals 2021, 14, 414. [Google Scholar] [CrossRef]
- Li, S.; Pi, J.; Zhu, H.; Yang, L.; Zhang, X.; Ding, W. Caffeic acid in tobacco root exudate defends tobacco plants from infection by Ralstonia solanacearum. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 2019, 178, 176–189.e15. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Trovato, M. Proline affects flowering time in Arabidopsis by modulating FLC expression: A clue of epigenetic regulation? Plants 2022, 11, 2348. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid–and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, M.J.; Jin, D.C.; Park, S.N.; Cho, E.G.; Freire, M.O.; Jang, S.J.; Park, Y.J.; Kook, J.K. Antimicrobial effect of ursolic acid and oleanolic acid against methicillin-resistant Staphylococcus aureus. Korean J. Microbiol. 2012, 48, 212–215. [Google Scholar] [CrossRef]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Rodin, J.O.; Silverstein, R.M.; Burkholder, W.E.; Gorman, J.E. Sex attractant of female dermestid beetle Trogoderma inclusum Le Conte. Science 1969, 165, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.L.; Bowers, M.D.; Irwin, R.E. Nectar chemistry mediates the behavior of parasitized bees: Consequences for plant fitness. Ecology 2016, 97, 325–337. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Sheng, M.; Chen, G.; Aldrich, J.R.; Chauhan, K.R. Iridodial: A powerful attractant for the green lacewing, Chrysopa septempunctata (Neuroptera: Chrysopidae). Naturwissenschaften 2006, 93, 461–465. [Google Scholar] [CrossRef]
- Melo, N.; Capek, M.; Arenas, O.M.; Afify, A.; Yilmaz, A.; Potter, C.J.; Laminette, P.J.; Para, A.; Gallio, M.; Stensmyr, M.C. The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr. Biol. 2021, 31, 1988–1994.e5. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.R.; Dayan, F.E.; Mamonov, L.K.; Mukanova, G.S.; Sitpaeva, G.T.; Gemejieva, N.G. Composition and phytotoxic activity of Nepeta pannonica L. essential oil. J. Essent. Oil Res. 2005, 17, 704–707. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D. Chemoinformatics approach to antibacterial studies of essential oils. Nat. Prod. Commun. 2015, 10, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Nararak, J.; Giorgio, C.D.; Sukkanon, C.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-repellency and biological safety of β-caryophyllene oxide against Aedes albopictus and Anopheles dirus (Diptera: Culicidae). Acta Trop. 2020, 210, 105556. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanim, K.A.; Krishnappa, K.; Pandiyan, J.; Nicoletti, M.; Gurunathan, B.; Govindarajan, M. Insecticidal potential of Matricaria chamomilla’s essential oil and its components (E)-β-farnesene, germacrene D, and α-bisabolol oxide A against agricultural pests, malaria, and Zika virus vectors. Agriculture 2023, 13, 779. [Google Scholar] [CrossRef]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B.; et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, M.; Lu, Y.; Zhang, W.; Sun, J.; Zeng, R.; Turlings, T.C.J.; Chen, L. A trail pheromone mediates the mutualism between ants and aphids. Curr. Biol. 2021, 31, 4738–4747.e4. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Stanković-Jeremić, J.; Cvetković, M. Phyto-pharmacological aspects of Nepeta nuda L.: A systematic review. Nat. Med. Mater. 2020, 40, 75–83. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Morán Vieyra, F.E.; Mercadante, A.Z.; Borsarelli, C.D. Singlet oxygen quenching by anthocyanin’s flavylium cations. Free Radic Res. 2008, 42, 885–891. [Google Scholar] [CrossRef]
- Khatri, D.; Chhetri, S.B.B. Reducing sugar, total phenolic content, and antioxidant potential of Nepalese plants. Biomed Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Hecht, A.L.; Harling, L.C.; Friedman, E.S.; Tanes, C.; Lee, J.; Firrman, J.; Tu, V.; Liu, L.S.; Bittinger, K.; Goulian, M.; et al. Colonization and dissemination of Klebsiella pneumoniae is dependent on dietary carbohydrates. bioRxiv 2023. bioRxiv:2023.05.25.542283. [Google Scholar] [CrossRef]
- Chen, F.; Elgaher, W.A.M.; Winterhoff, M.; Büssow, K.; Waqas, F.H.; Graner, E.; Pires-Afonso, Y.; Casares Perez, L.; de la Vega, L.; Sahini, N.; et al. Citraconate inhibits ACOD1 (IRG1) catalysis, reduces interferon responses and oxidative stress, and modulates inflammation and cell metabolism. Nat. Metab. 2022, 4, 534–546. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef]
- Lin, T.H.; Huang, S.H.; Wu, C.C.; Liu, H.H.; Jinn, T.R.; Chen, Y.; Lin, C.T. Inhibition of Klebsiella pneumoniae growth and capsular polysaccharide biosynthesis by Fructus mume. Evid.-Based Complement. Altern. Med. 2013, 2013, 621701. [Google Scholar] [CrossRef]
- Jenior, M.L.; Dickenson, M.E.; Papin, J.A. Genome-scale metabolic modeling reveals increased reliance on valine catabolism in clinical isolates of Klebsiella pneumoniae. NPJ Syst. Biol. Appl. 2022, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, S.; Zhao, X.; Zhou, S. l-Proline protects mice challenged by Klebsiella pneumoniae bacteremia. J. Microbiol. Immunol. Infect. 2021, 54, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tawfeeq, H.K. The effect of D and L-amino acids on biofilm formation in different microorganisms. Iraqi J. Sci. 2016, 57, 570–575. [Google Scholar]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van den Ende, W. Fructans as Immunomodulatory and Antiviral Agents: The Case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Pająk, B.; Zieliński, R.; Manning, J.T.; Matejin, S.; Paessler, S.; Fokt, I.; Emmett, M.R.; Priebe, W. The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses. Molecules 2022, 27, 5928. [Google Scholar] [CrossRef]
- Mei, R.; Heng, X.; Liu, X.; Chen, G. Glycopolymers for antibacterial and antiviral applications. Molecules 2023, 28, 985. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Q.; Yi, Y.; Xiao, H.; Putra, D.F.; Ke, K.; Zhang, Q.; Li, P. Antiviral activities of Lonicera japonica Thunb. components against grouper iridovirus in vitro and in vivo. Aquaculture 2020, 519, 734882. [Google Scholar] [CrossRef]
- Nguyen, B.C.Q.; Shahinozzaman, M.; Tien, N.T.K.; Thach, T.N.; Tawata, S. Effect of sucrose on antioxidant activities and other health-related micronutrients in gamma-aminobutyric acid (GABA)-enriched sprouting Southern Vietnam brown rice. J. Cereal Sci. 2020, 93, 102985. [Google Scholar] [CrossRef]
- Ai, X.; He, W.; Wang, X.; Wang, Z.; Wang, G.; Lu, H.; Qin, S.; Li, Z.; Guan, J.; Zhao, K.; et al. Antiviral effect of lysosomotropic disaccharide trehalose on porcine hemagglutinating encephalomyelitis virus, a highly neurotropic betacoronavirus. Virology 2022, 577, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Fatoki, T.H.; Sanni, D.M.; Momodu, D.U.; Ugboko, H.U.; Adeseko, C.J.; Faleye, B.C. Evaluation of empirical functions and fate of isomaltose. J. Appl. Life Sci. Int. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Fang, L.; Gao, Y.; Lan, M.; Jiang, P.; Bai, J.; Li, Y.; Wang, X. Hydroquinone inhibits PRV infection in neurons in vitro and in vivo. Vet. Microbiol. 2020, 250, 108864. [Google Scholar] [CrossRef]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Stankova, I.; Chuchkov, K.; Shishkov, S.; Kostova, K.; Mukova, L.; Galabov, A.S. Synthesis, antioxidative and antiviral activity of hydroxycinnamic acid amides of thiazole containing amino acid. Amino Acids 2009, 37, 383–388. [Google Scholar] [CrossRef]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Bai, J.; Wu, Y.; Zhong, K.; Xiao, K.; Liu, L.; Huang, Y.; Wang, Z.; Gao, H. A Comparative study on the effects of quinic acid and shikimic acid on cellular functions of Staphylococcus aureus. J. Food Prot. 2018, 81, 1187–1192. [Google Scholar] [CrossRef]
- Hobby, C.R.; Herndon, J.L.; Morrow, C.A.; Peters, R.E.; Symes, S.J.K.; Giles, D.K. Exogenous fatty acids alter phospholipid composition, membrane permeability, capacity for biofilm formation, and antimicrobial peptide susceptibility in Klebsiella pneumoniae. MicrobiologyOpen 2019, 8, e00635. [Google Scholar] [CrossRef]
- Das, D.; Arulkumar, A.; Paramasivam, S.; Lopez-Santamarina, A.; del Carmen Mondragon, A.; Miranda Lopez, J.M. Phytochemical constituents, antimicrobial properties and bioactivity of marine red seaweed (Kappaphycus alvarezii) and seagrass (Cymodocea serrulata). Foods 2023, 12, 2811. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.B. Phytochemical investigation using GC/MS analysis and evaluation of antimicrobial and cytotoxic activities of the lipoidal matter of leaves of Sophora secundiflora and Sophora tomentosa. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 207–214. [Google Scholar] [CrossRef]
- Mint Evolutionary Genomics Consortium. Phylogenomic mining of the mints reveals multiple mechanisms contributing to the evolution of chemical diversity in Lamiaceae. Mol. Plant 2018, 11, 1084–1096. [Google Scholar] [CrossRef] [PubMed]





| Volatiles | RT | RI | In Vitro | Flower | Leaf | |
|---|---|---|---|---|---|---|
| V1 | β-Pinene | 10.18 | 1110.5 | 0.32 c | 1.85 b | 2.22 a |
| V2 | Sabinene | 10.53 | 1123.4 | 0.15 c | 0.84 b | 0.99 a |
| V3 | Myrcene | 11.75 | 1167.9 | 0.10 b | 0.54 a | 1.16 a |
| V4 | D-Limonene | 13.01 | 1209.9 | 0.05 c | 0.28 b | 0.35 a |
| V5 | 1,8-Cineole/Eucalyptol | 13.34 | 1218.3 | 3.75 b | 16.15 a | 18.44 a |
| V6 | 2-Hexenal | 13.98 | 1230.5 | 0.00 b | 0.00 b | 0.84 а |
| V7 | trans-β-Ocimene | 14.21 | 1242.2 | 0.06 b | 0.00 c | 0.38 a |
| V8 | β-Ocimene | 14.87 | 1259.0 | 0.14 b | 0.13 b | 1.79 a |
| V9 | 3-Octanone | 15.19 | 1261.6 | 0.00 c | 0.07 b | 0.42 a |
| V10 | Benzene, m-di-tert-butyl- | 22.03 | 1435.4 | 0.00 c | 0.08 b | 0.12 a |
| V11 | 1-Octen-3-ol | 22.95 | 1457.8 | 0.00 c | 0.27 b | 0.96 a |
| V12 | γ-Elemene | 23.99 | 1489.8 | 0.06 c | 0.19 b | 0.65 a |
| V13 | β-Bourbonene | 25.74 | 1527.1 | 0.00 b | 0.48 a | 0.46 a |
| V14 | Camphor | 25.87 | 1530.3 | 0.00 c | 0.18 b | 0.32 a |
| V15 | α-Gurjunene | 25.99 | 1539.0 | 0.05 c | 0.37 b | 1.34 a |
| V16 | β-Copaene | 27.74 | 1582.4 | 0.25 c | 0.52 b | 0.87 a |
| V17 | β-Elemene | 28.40 | 1598.7 | 0.42 c | 0.85 b | 1.52 a |
| V18 | Caryophyllene | 28.71 | 1606.4 | 2.85 b | 2.36 c | 6.07 a |
| V19 | Humulene | 31.52 | 1680.8 | 0.56 b | 0.46 c | 1.20 a |
| V20 | δ-Terpineol | 31.67 | 1684.9 | 0.10 b | 0.48 a | 0.49 a |
| V21 | α-Terpineol | 32.61 | 1709.7 | 0.25 b | 1.18 a | 1.17 a |
| V22 | Germacrene D | 33.02 | 1720.6 | 4.92 c | 7.70 b | 13.61 a |
| V23 | Bicyclogermacren | 33.95 | 1745.2 | 0.16 c | 0.71 b | 2.32 a |
| V24 | Benzothiazole | 42.21 | 1977.8 | 0.30 a | 0.00 b | 0.00 b |
| V25 | Ledol | 44.58 | 2042.4 | 0.00 b | 0.00 b | 0.54 a |
| V26 | 4a-α,7-β,7a-α-Nepetalactone | 45.50 | 2078.8 | 64.41 a | 46.13 b | 0.00 c |
| Bioactivities | Unit | In Vitro | Flower | Leaf | Reference | |
|---|---|---|---|---|---|---|
| AO | Antioxidant (DPPH) | mM g−1DW | 60.81 c | 206.00 a | 75.63 b | [10] |
| AHHV1SA | Anti-human herpes virus 1 simultaneous application | % inhibition | 0.00 b | 80.33 a | 75.67 a | [10] |
| AHHV1PA | Anti-human herpes virus 1 postinfection application | % inhibition | 0.00 c | 64.76 a | 43.52 b | [10] |
| AI | Anti-inflammatory | % inhibition | 4.64 c | 51.31 a | 44.13 b | This study |
| ASa | Anti-bacterial (Gram+) | inhibition zone mm | 10.00 a | 10.67 a | 10.00 a | [10] |
| AKp | Anti-bacterial (Gram−) | inhibition zone mm | 10.67 a | 12.67 a | 8.00 a | [10] |
| Active Hormones | In Vitro | Flower | Leaf |
|---|---|---|---|
| Cytokinins (CKs) | 6.76 a | 5.79 a | 6.03 a |
| Gibberellins (GA19) | 4.44 a | 0.62 b | 1.72 ab |
| Abscisic acid (ABA) | 34.59 b | 662.25 a | 696.19 a |
| Jasmonic acid (JA) | 180.64 c | 1773.33 a | 363.66 b |
| Auxin (IAA, indole-3-acetic acid) | 29.16 c | 305.45 a | 95.04 b |
| Salicylic acid (SA) | 146.22 c | 1020.39 a | 596.59 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, A.; Rusanov, K.; Rusanova, M.; Paunov, M.; Yordanova, Z.; Mantovska, D.; Tsacheva, I.; Petrova, D.; Mishev, K.; Dobrev, P.I.; et al. Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.). Metabolites 2023, 13, 1099. https://doi.org/10.3390/metabo13101099
Zaharieva A, Rusanov K, Rusanova M, Paunov M, Yordanova Z, Mantovska D, Tsacheva I, Petrova D, Mishev K, Dobrev PI, et al. Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.). Metabolites. 2023; 13(10):1099. https://doi.org/10.3390/metabo13101099
Chicago/Turabian StyleZaharieva, Anna, Krasimir Rusanov, Mila Rusanova, Momchil Paunov, Zhenya Yordanova, Desislava Mantovska, Ivanka Tsacheva, Detelina Petrova, Kiril Mishev, Petre I. Dobrev, and et al. 2023. "Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.)" Metabolites 13, no. 10: 1099. https://doi.org/10.3390/metabo13101099
APA StyleZaharieva, A., Rusanov, K., Rusanova, M., Paunov, M., Yordanova, Z., Mantovska, D., Tsacheva, I., Petrova, D., Mishev, K., Dobrev, P. I., Lacek, J., Filepová, R., Zehirov, G., Vassileva, V., Mišić, D., Motyka, V., Chaneva, G., & Zhiponova, M. (2023). Uncovering the Interrelation between Metabolite Profiles and Bioactivity of In Vitro- and Wild-Grown Catmint (Nepeta nuda L.). Metabolites, 13(10), 1099. https://doi.org/10.3390/metabo13101099