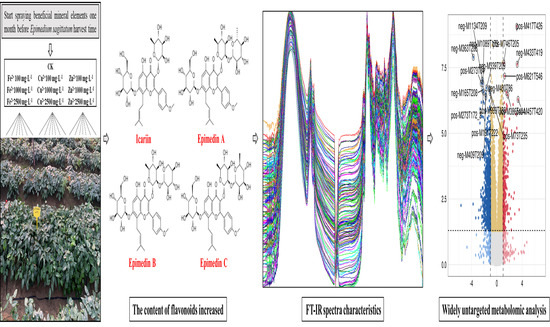
A Comprehensive Analysis to Elucidate the Effects of Spraying Mineral Elements on the Accumulation of Flavonoids in Epimedium sagittatum during the Harvesting Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatment Methods
2.2. Determination of the Four Flavonoids’ Contents in E. sagittatum Leaves by HPLC
2.3. Establishment of FT-IR Spectra of E. sagittatum Leaf Samples
2.4. Widely Untargeted Metabolomic Analysis of E. sagittatum Leaves
2.5. Statistical Analysis
3. Results
3.1. The Four Flavonoids’ Contents in E. sagittatum Leaves
3.2. The FT-IR Spectra Characteristics in E. sagittatum Leaves
3.3. The OPLS-DA Analysis of FT-IR Characteristic Spectra in E. sagittatum Leaves
3.4. Widely Untargeted Metabolomic Analysis of E. sagittatum Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.-J.; Tang, Z.-H.; Li, X.-W.; Xie, C.-X.; Lu, J.-J.; Wang, Y.-T. Chemical constituents, quality control, and bioactivity of Epimedii Folium (Yinyanghuo). Am. J. Chin. Med. 2015, 43, 783–834. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Wei, G.; Liao, S.; Zhang, Y.; Huang, W.; Yuan, L.; Wang, Y. Chemotypic and genetic diversity in Epimedium sagittatum from different geographical regions of China. Phytochemistry 2015, 116, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Chen, J.; Sun, W.; Wang, Y. Taxonomic and phylogenetic analysis of Epimedium L. based on amplified fragment length polymorphisms. Sci. Hortic. 2014, 170, 284–292. [Google Scholar] [CrossRef]
- Li, W.-X.; Deng, Y.-Y.; Li, F.; Liu, B.; Liu, H.-Y.; Shi, J.-S.; Gong, Q.-H. Icariin, a major constituent of flavonoids from Epimedium brevicornum, protects against cognitive deficits induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. Pharmacol. Biochem. Behav. 2015, 138, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lien, E.J.; Lien, L.L. Chemical and pharmacological investigations of Epimedium species: A survey. In Progress in Drug Research; Wu, H., Lien, E.J., Lien, L.L., Schultz, R.M., Ram, V.J., Domingo, E., Spence, P., Gupta, S.P., Bhat, S.P., Villarreal, E.C., et al., Eds.; Birkhäuser Basel: Basel, Switzerland, 2003; pp. 1–57. [Google Scholar]
- Jiang, J.; Song, J.; Jia, X.-B. Phytochemistry and ethnopharmacology of Epimedium L. Species. Chin. Herb. Med. 2015, 7, 204–222. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Zhang, X.; Yang, X.-H.; Qiu, N.-X.; Wang, Y.; Wang, Z.-Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Shen, P.; Guo, B.L.; Gong, Y.; Hong, D.Y.Q.; Hong, Y.; Yong, E.L. Taxonomic, genetic, chemical and estrogenic characteristics of Epimedium species. Phytochemistry 2007, 68, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q.; Xu, J.-M.; Liang, J.; Cheng, Y.; Li, S.; Zheng, L.; Ye, B.; Meng, K.; Qin, S. A multicenter, single arm phase II trial of a small molecule immune-modulator icaritin: Safety, overall survival, immune dynamics, and PD-L1 expression in advanced hepatocellular carcinoma. J. Clin. Oncol. 2018, 36 (Suppl. S15), 4077. [Google Scholar] [CrossRef]
- Mo, D.; Zhu, H.; Wang, J.; Hao, H.; Guo, Y.; Wang, J.; Han, X.; Zou, L.; Li, Z.; Yao, H.; et al. Icaritin inhibits PD-L1 expression by Targeting Protein IκB Kinase α. Eur. J. Immunol. 2021, 51, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, G.; Pieters, S.; Logie, H.; Van Erps, J.; Merino-Arévalo, M.; Dejaegher, B.; Smeyers-Verbeke, J.; Vander Heyden, Y. Exploration and classification of chromatographic fingerprints as additional tool for identification and quality control of several Artemisia species. J. Pharm. Biomed. Anal. 2014, 95, 34–46. [Google Scholar] [CrossRef]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef]
- Huang, W.; Zeng, S.; Xiao, G.; Wei, G.; Liao, S.; Chen, J.; Sun, W.; Lv, H.; Wang, Y. Elucidating the biosynthetic and regulatory mechanisms of flavonoid-derived bioactive components in Epimedium sagittatum. Front. Plant Sci. 2015, 6, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.; Singh, A. Chapter 19—Biofertilizers: “An ace in the hole” in medicinal and aromatic plants cultivation. In Biofertilizers; Rakshit, A., Meena, V.S., Parihar, M., Singh, H.B., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 253–263. [Google Scholar]
- Toksha, B.; Sonawale, V.A.M.; Vanarase, A.; Bornare, D.; Tonde, S.; Hazra, C.; Kundu, D.; Satdive, A.; Tayde, S.; Chatterjee, A. Nanofertilizers: A review on synthesis and impact of their use on crop yield and environment. Environ. Technol. Innov. 2021, 24, 101986. [Google Scholar] [CrossRef]
- Bayram, S.E.; İsfendiyaroğlu, M.; Tuncay, Ö. Effects of foliar zinc applications on some yield parameters and essential oil constituents of the mastic tree (Pistacia lentiscus var. chia Duham.). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100431. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Wang, Q.; Sun, Y.; Duan, N.; Chen, Z.; Zeng, H. Spray treatment of leaves with Fe2+ promotes procyanidin biosynthesis by upregulating the expression of the F3H and ANS genes in red rice grains (Oryza sativa L.). J. Cereal Sci. 2021, 100, 103231. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, S.; Hou, Y.; Ji, B.; Pei, L.; Su, X.; Zhong, H.; Dong, C. Blue light induces biosynthesis of flavonoids in Epimedium sagittatum (Sieb.et Zucc.) Maxim. leaves, a study on a light-demanding medicinal shade herb. Ind. Crops Prod. 2022, 187, 115512. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [Green Version]
- Pei, L.-K.; Sun, S.-Q.; Guo, B.-L.; Huang, W.-H.; Xiao, P.-G. Fast quality control of Herba Epimedii by using Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Zuo, Z.; Wang, Y. Determination of total flavonoids for Paris polyphylla var. yunnanensis in different geographical origins using UV and FT-IR spectroscopy. J. AOAC Int. 2019, 102, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-M.; Zha, Q.-L.; Chen, T.-B.; Xiao, S.-Y.; Xie, Y.; Luo, P.; Wang, Y.-P.; Liu, L.; Zhou, H. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC-QTOF/MS coupled with OPLS-DA. Phytomedicine 2018, 45, 8–17. [Google Scholar] [CrossRef]
- Singh, P.A.; Bajwa, N.; Chinnam, S.; Chandan, A.; Baldi, A. An overview of some important deliberations to promote medicinal plants cultivation. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100400. [Google Scholar] [CrossRef]
- Kumar, D.; Punetha, A.; Verma, P.P.S.; Padalia, R.C. Micronutrient based approach to increase yield and quality of essential oil in aromatic crops. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100361. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Wang, Y.; Gao, Y.; Zhang, L. The response of ginseng grown on farmland to foliar-applied iron, zinc, manganese and copper. Ind. Crops Prod. 2013, 45, 388–394. [Google Scholar] [CrossRef]
- Rostami, M.; Talarposhti, R.M.; Mohammadi, H.; Demyan, M.S. Morpho-physiological Response of Saffron (Crocus sativus L.) to Particle Size and Rates of Zinc Fertilizer. Commun. Soil Sci. Plant Anal. 2019, 50, 1250–1257. [Google Scholar] [CrossRef]
- Ghatas, Y.; Ali, M.; Elsadek, M.; Mohamed, Y. Enhancing growth, productivity and artemisinin content of Artemisia annua L. Plant using seaweed extract and micronutrients. Ind. Crops Prod. 2021, 161, 113202. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S. Chapter 2—The importance of beneficial and essential trace and ultratrace elements in plant nutrition, growth, and stress tolerance. In Plant Nutrition and Food Security in the Era of Climate Change; Kumar, V., Srivastava, A.K., Suprasanna, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 27–46. [Google Scholar]
- Li, P.; Wang, A.; Du, W.; Mao, L.; Wei, Z.; Wang, S.; Yuan, H.; Ji, R.; Zhao, L. Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level. Sci. Total Environ. 2020, 738, 139795. [Google Scholar] [CrossRef] [PubMed]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef]
- Afzal, S.; Singh, N.K.; Singh, N.; Sohrab, S.; Rani, M.; Mishra, S.K.; Agarwal, S.C. Chapter 9—Effect of metals and metalloids on the physiology and biochemistry of medicinal and aquatic plants. In Metals Metalloids Soil Plant Water Systems; Aftab, T., Hakeem, K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 199–216. [Google Scholar]
- Esteki, M.; Memarbashi, N.; Simal-Gandara, J. Classification and authentication of tea according to their harvest season based on FT-IR fingerprinting using pattern recognition methods. J. Food Compos. Anal. 2022, 115, 104995. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Kumar, J.; Nikam, T.D.; Suprasanna, P. FT-IR profiling reveals differential response of roots and leaves to salt stress in a halophyte Sesuvium portulacastrum (L.). Biotechnol. Rep. 2019, 23, e00352. [Google Scholar] [CrossRef]
- He, W.; Lei, T. Identification of camellia oil using FT-IR spectroscopy and chemometrics based on both isolated unsaponifiables and vegetable oils. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117839. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Wang, C.-J.; Liang, J.; Lao, C.-C.; Zhou, H.; Zhang, Z.-F. A new approach for identification of medicinal almonds by fourier transform infrared spectroscopy and systematic clustering of characteristic peaks. Chin. J. Nat. Med. 2017, 15, 703–709. [Google Scholar] [CrossRef]
- Shannon, M.; Lafeuille, J.-L.; Frégière-Salomon, A.; Lefevre, S.; Galvin-King, P.; Haughey, S.A.; Burns, D.T.; Shen, X.; Kapil, A.; McGrath, T.F.; et al. The detection and determination of adulterants in turmeric using fourier-transform infrared (FTIR) spectroscopy coupled to chemometric analysis and micro-FTIR imaging. Food Control. 2022, 139, 109093. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Yeap, Z.Q.; Zhang, X.; Wu, S.; Ng, C.H.; Yam, M.F. Rapid authentication and identification of different types of A. roxburghii by Tri-step FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 271–282. [Google Scholar] [CrossRef]
- Chen, J.; Guo, B.; Yan, R.; Sun, S.; Zhou, Q. Rapid and automatic chemical identification of the medicinal flower buds of Lonicera plants by the benchtop and hand-held Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 182, 81–86. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Li, C.-G.; Chang, D.; Bensoussan, A. Current status and major challenges to the safety and efficacy presented by Chinese herbal medicine. Medicines 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scossa, F.; Benina, M.; Alseekh, S.; Zhang, Y.; Fernie, A.R. The integration of metabolomics and next-generation sequencing data to elucidate the pathways of natural product metabolism in medicinal plants. Planta Med. 2018, 84, 855–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3662. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, F.; Yan, Y.; Gu, X.; Zhou, S.; Su, X.; Ji, B.; Zhong, H.; Dong, C. A Comprehensive Analysis to Elucidate the Effects of Spraying Mineral Elements on the Accumulation of Flavonoids in Epimedium sagittatum during the Harvesting Period. Metabolites 2023, 13, 294. https://doi.org/10.3390/metabo13020294
Yang L, Zhang F, Yan Y, Gu X, Zhou S, Su X, Ji B, Zhong H, Dong C. A Comprehensive Analysis to Elucidate the Effects of Spraying Mineral Elements on the Accumulation of Flavonoids in Epimedium sagittatum during the Harvesting Period. Metabolites. 2023; 13(2):294. https://doi.org/10.3390/metabo13020294
Chicago/Turabian StyleYang, Linlin, Fei Zhang, Yueci Yan, Xupeng Gu, Shengwei Zhou, Xiuhong Su, Baoyu Ji, Hua Zhong, and Chengming Dong. 2023. "A Comprehensive Analysis to Elucidate the Effects of Spraying Mineral Elements on the Accumulation of Flavonoids in Epimedium sagittatum during the Harvesting Period" Metabolites 13, no. 2: 294. https://doi.org/10.3390/metabo13020294
APA StyleYang, L., Zhang, F., Yan, Y., Gu, X., Zhou, S., Su, X., Ji, B., Zhong, H., & Dong, C. (2023). A Comprehensive Analysis to Elucidate the Effects of Spraying Mineral Elements on the Accumulation of Flavonoids in Epimedium sagittatum during the Harvesting Period. Metabolites, 13(2), 294. https://doi.org/10.3390/metabo13020294