GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants
Abstract
:1. Introduction
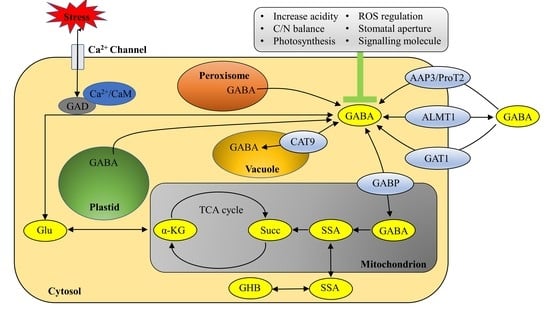
2. Distribution of GABA in Plant Cells
3. GABA Biosynthesis and Catabolism in Plants
3.1. Biosynthesis of GABA from Glutamate Decarboxylation, Polyamine Degradation and Proline Nonenzymatic Conversion
3.1.1. Glutamate Decarboxylation
3.1.2. Polyamine Degradation
3.1.3. Polyamine Degradation
3.2. GABA Catabolism Generates Succinate and γ- Hydroxybutyric Acid (GHB)
3.2.1. GABA Is Converted to Succinate
3.2.2. GABA Is Converted to GHB
| Type | Gene | Species | Description |
|---|---|---|---|
| Biosynthesis | GAD1 [46] | Arabidopsis, Tomato, Citrus, Poplar, Tea | In Arabidopsis, it affects the GABA level of roots. In tomato, it promotes fruit growth and development but has no significant correlation with GABA. In citrus and tea, it promotes the accumulation of GABA; in poplar, there are auxin, ABA and gibberellin response elements |
| GAD2 [84] | Arabidopsis, Tomato, Citrus, Rice, Tobacco, Poplar, Tea | In Arabidopsis, it mainly affects the GABA level in the shoot but does not affect the GABA level in the root. In tomato, rice, citrus, tea and tobacco, the expression of the GAD2 gene is significantly increased, which increased the content of GABA; in poplar, there are gibberellin and ABA response elements | |
| GAD3 [84] | Arabidopsis, Tomato, Tobacco, Poplar, Tea | In Arabidopsis, it has no C-terminal domain, is not regulated by Ca2+, and is expressed in young leaves and immature fruits. In tomato, tea, poplar and tobacco, GABA level can be increased | |
| GAD4 [47] | Arabidopsis, Tomato, Poplar | In Arabidopsis, there is no effect on GABA level. In tomato, it has nothing to do with plant growth and development; in poplar, there are gibberellin response elements | |
| GAD5 [85] | Arabidopsis, Poplar | It has no C-terminal domain and is not regulated by Ca2+ and is mainly expressed in flowers; in poplar, there are ABA response elements | |
| GAD6 [86] | Poplar | There are gibberellin and ABA response elements | |
| DAOs [87] | Arabidopsis. Soybean, Peanut, Broad bean | It can oxidize Put, Spm and Spd; Cu2+ can activate the activity, but EDTA treatment can reduce the activity; mainly distributed in dicotyledonous plants such as legumes; Arabidopsis contains 10 CuAOs coding genes | |
| PAOs [57] | Arabidopsis, Tea, Rice, Maize, Wheat | It can oxidize Spm and Spd; FAD can be used as its coenzyme; mainly distributed in monocotyledonous plants such as cereals; Arabidopsis contains five polyamine oxidase genes (AtPAO1-5); tea contains seven PAO genes (CsPAO1-7) | |
| Catabolism | POP2 [63] | Arabidopsis | The production of functional GABA-T enzyme ensures the GABA gradient required to guide the growth of pollen tubes in the pistil and then regulate the development of roots and shoots |
| GABA-T1 [88] | Tomato, Poplar | In tomato, it mainly exists in mitochondria and is highly expressed, promoting the catabolism of GABA and avoiding plant dwarfing and sterility; in poplar, the expression of genes is low in leaves and increases in stems and roots in turn | |
| GABA-T2 [88] | Tomato, Poplar | In tomato, it is mainly located in the cytoplasm to regulate GABA catabolism; in poplar, there is no significant difference in gene expression between leaves and stems but high expression in roots | |
| GABA-T3 [80] | Tomato | Mainly expressed in plastids to promote the catabolism of GABA; ensuring the normal growth and development of plants | |
| SSADH1 [17] | Arabidopsis, Tomato, Poplar | Promoting the conversion of SSA to succinate; in Arabidopsis, small size necrotic lesions of plants are avoided, and GHB production is promoted; there is no correlation with GABA content in tomato; in poplar, there are light response, gibberellin and response elements involved in anaerobic induction | |
| SSADH2 [89] | Poplar | In poplar, the expression of response elements containing light response and gibberellin in leaves, stems and roots increased in turn | |
| SSR1 [90] | Tomato | Promoting the conversion from SSA to GHB; it exists in the cytoplasm and has a high expression level at maturity | |
| SSR2 [90] | Tomato | Promoting the conversion from SSA to GHB; it exists in mitochondria and plastids, and its expression level is high at the stage of color breaking |
4. Transport of Exogenous GABA in Plants
4.1. Transcell Membrane GABA Transporters
4.1.1. ALMT1
4.1.2. GAT1
4.1.3. AAP3 and ProT2
4.2. Transorganelle Membrane GABA Transporter
4.2.1. BAT1
| Type | Transporter | Species | Description |
|---|---|---|---|
| Cell membrane | ALMT1 [100] | Arabidopsis, Wheat, Barley, Rice | Trans-cell membrane transport of GABA between apoplast and cytoplasm. Anions can activate its activity, and Al3+ can promote GABA efflux through it. GABA inhibits the transport of anions in wheat by changing the active structure of ALMT1 |
| GAT1 [104] | Arabidopsis, Rice, Potato | A high-affinity GABA transporter protein, which transports GABA from the apoplast to the cytoplasm; the GAT1 gene belongs to the AAAP gene family | |
| AAP3 [91] | Arabidopsis, Rice, Potato | The affinity for GABA is lower than other amino acids, such as lysine; the AAP3 gene belongs to the AAAP family | |
| ProT2 [111] | Arabidopsis, Rice, Potato | Having higher affinity for compatible solutions of proline and glycine betaine than GABA; the ProT2 gene belongs to the ATF superfamily | |
| Organelle membrane | CAT9 [95] | Arabidopsis, Tomato, Rice, Potato | Experimental verification of GABA transport function of related gene (SlCAT9) in tomato; the CAT9 gene belongs to the APC gene family; transport through gradient concentration of substrate and driving force of vacuolar membrane proton pump |
| GABP [114] | Arabidopsis | AtGABP (At2g01170.1) is a splicing variant of AtBAT1 (At2g01170) belonging to the APC gene family; coexpression of GABP and SSADH |
4.2.2. CAT9
5. Function and Mechanism of GABA in the Regulation of the Abiotic Stress Response in Plants
5.1. Hypoxic Stress
5.1.1. GABA Accumulation under Hypoxic Stress
5.1.2. Function of GABA under Hypoxic Stress
5.2. Salt Stress
5.2.1. GABA Accumulation under Salt Stress
5.2.2. Function of GABA under Salt Stress
5.3. Drought Stress
5.3.1. Gaba Accumulation under Drought Stress
5.3.2. Function of GABA under Drought Stress
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. Gamma-aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Steward, F.C.; Thompson, J.F.; Dent, C.E. γ-Aminobutyric acid: A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Bown, A.W.; Shelp, B.J. The metabolism and functions of γ-aminobutyric acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bown, A.W.; Shelp, B.J. Does the GABA shunt regulate cytosolic GABA? Trends Plant Sci. 2020, 25, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Fromm, H. GABA signaling in plants: Targeting the missing pieces of the puzzle. J. Exp. Bot. 2020, 71, 6238–6245. [Google Scholar] [CrossRef]
- Frank, L.; Anke, H.; Hillel, F.; Linda, B.; Nicolas, B.; Markus, G. Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis. PLoS ONE 2008, 3, e3383. [Google Scholar]
- Gramazio, P.; Takayama, M.; Ezura, H. Challenges and prospects of new plant breeding techniques for GABA improvement in crops: Tomato as an example. Front. Plant Sci. 2020, 11, 577980. [Google Scholar] [CrossRef]
- Khan, M.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Peng, L.; Wu, X.; Gong, B.; Li, J.; Lü, G.; Gao, H. Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress. Antioxidants 2022, 11, 2106. [Google Scholar]
- Yang, R.; Guo, Y.; Wang, S.; Gu, Z. Ca2+ and aminoguanidine on gamma-aminobutyric acid accumulation in germinating soybean under hypoxia-NaCl stress. J. Food Drug Anal. 2015, 23, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Jianhua, L.; Umair, A.; Gaoke, L.; Yuliang, L.; Wenjia, L. Exogenous gamma-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in Maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar]
- Mekonnen, D.W.; Flügge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef] [Green Version]
- Sheng, L.; Shen, D.; Yang, W.; Zhang, M.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. GABA pathway rate-limit citrate degradation in postharvest citrus fruit evidence from HB Pumelo (Citrus grandis) x Fairchild (Citrus reticulata) hybrid population. J. Agric. Food Chem. 2017, 65, 1669–1676. [Google Scholar] [CrossRef]
- Ma, H. Plant reproduction: GABA gradient, guidance and growth. Curr. Biol. 2003, 13, R834–R836. [Google Scholar] [CrossRef] [Green Version]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V. Diverse role of gamma-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Yu, P.; Ren, Q.; Wang, X.; Huang, X. Enhanced biosynthesis of γ-aminobutyric acid (GABA) in Escherichia coli by pathway engineering. Biochem. Eng. J. 2019, 141, 252–258. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate decarboxylase from lactic acid bacteria-A key enzyme in GABA synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Shen, Q.; Li, R.; Cao, Y.; Li, Y.; Zou, Z.; Ren, T.; Li, F. GABA shunt contribution to flavonoid biosynthesis and metabolism in tea plants (Camellia sinensis). Plant Physiol. Biochem. 2021, 166, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Breitkreuz, K.E.; Allan, W.L.; Van Cauwenberghe, O.R.; Jakobs, C.; Talibi, D.; Andre, B.; Shelp, B.J. A novel gamma-hydroxybutyrate dehydrogenase: Identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. J. Biol. Chem. 2003, 278, 41552–41556. [Google Scholar] [CrossRef] [Green Version]
- Allan, W.L.; Simpson, J.P.; Clark, S.M.; Shelp, B.J. Gamma-hydroxybutyrate accumulation in Arabidopsis and tobacco plants is a general response to abiotic stress: Putative regulation by redox balance and glyoxylate reductase isoforms. J. Exp. Bot. 2008, 59, 2555–2564. [Google Scholar] [CrossRef] [Green Version]
- McCraw, S.L.; Park, D.H.; Jones, R.; Bentley, M.A.; Rico, A.; Ratcliffe, R.G.; Kruger, N.J.; Collmer, A.; Preston, G.M. GABA (gamma-aminobutyric acid) uptake via the GABA permease GabP represses virulence gene expression in Pseudomonas syringae pv. tomato DC3000. Mol. Plant. Microbe Interact. 2016, 29, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [Green Version]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. N. Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Johnson, C.; Hall, J.L.; Ho, L.C. Pathways of uptake and accumulation of sugars in tomato fruit. Ann. Bot. 1988, 61, 593–603. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Missihoun, T.D.; Schmitz, J.; Klug, R.; Kirch, H.H.; Bartels, D. Betaine aldehyde dehydrogenase genes from Arabidopsis with different sub-cellular localization affect stress responses. Planta 2011, 233, 69–82. [Google Scholar] [CrossRef]
- Stiti, N.; Missihoun, T.D.; Kotchoni, S.O.; Kirch, H.H.; Bartels, D. Aldehyde dehydrogenases in Arabidopsis thaliana: Biochemical requirements, metabolic pathways, and functional analysis. Front. Plant Sci. 2011, 2, 65. [Google Scholar] [CrossRef] [Green Version]
- Aleksza, D.; Horváth, G.V.; Sándor, G.; Szabados, L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Bouchez, D.; Møller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Ruiz, R.; Felix, M.; Gertrude, K. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Behera, S.; Zhaolong, X.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; DeMichelis, M.I.; Morris, R.J.; Schwarzländer, M.; Costa, A. Cellular Ca2+ Signals generate defined pH signatures in plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [Green Version]
- Villand, P.; Olsen, O.A.; Kleczkowski, L.A. Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol. Biol. 1993, 23, 1279–1284. [Google Scholar] [CrossRef]
- Gut, H.; Dominici, P.; Pilati, S.; Astegno, A.; Petoukhov, M.V.; Svergun, D.I.; Grütter, M.G.; Capitani, G. A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 2009, 392, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Zik, M.; Arazi, T.; Snedden, W.A.; Fromm, H. Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol. Biol. 1998, 37, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2018, 95, 11. [Google Scholar] [CrossRef] [Green Version]
- Takayama, M.; Ezura, H. How and why does tomato accumulate a large amount of GABA in the fruit? Front. Plant Sci. 2015, 6, 612. [Google Scholar] [CrossRef] [Green Version]
- Bouché, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef]
- Baum, G.; Chen, Y.; Arazi, T.; Takatsuji, H.; Fromm, H. A plant glutamate decarboxylase containing a calmodulin binding domain. cloning, sequence, and functional analysis. J. Biol. Chem. 1993, 268, 19610–19617. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef]
- Trobacher, C.P.; Clark, S.M.; Bozzo, G.G.; Mullen, R.T.; Shelp, B.J. Catabolism of GABA in apple fruit: Subcellular localization and biochemical characterization of two γ-aminobutyrate transaminases. Postharvest Biol. Technol. 2012, 75, 106–113. [Google Scholar] [CrossRef]
- Akçay, N.; Bor, M.; Karabudak, T.; Ozdemir, F.; Türkan, I. Contribution of Gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J. Plant Physiol. 2012, 169, 452–458. [Google Scholar] [CrossRef]
- Ji, J.; Zheng, L.; Yue, J.; Yao, X.; Chang, E.; Xie, T.; Deng, N.; Chen, L.; Huang, Y.; Jiang, Z.; et al. Identification of two CiGADs from Caragana intermedia and their transcriptional responses to abiotic stresses and exogenous abscisic acid. PeerJ 2017, 5, e3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Zhao, L.; Li, Q.; Jiang, L.; Wan, J.M. Molecular cloning and expression of a novel glutamate decarboxylase gene in rice. Rice Genet. 2004, 21, 39–42. [Google Scholar]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Torrigiani, P.; Scoccianti, V.; Bagni, N. Polyamine oxidase activity and polyamine content in maize during seed germination. Physiol. Plantarum. 2010, 74, 427–432. [Google Scholar] [CrossRef]
- Flores, H.E.; Filner, P. Polyamine catabolism in higher plants: Characterization of pyrroline dehydrogenase. Plant Growth Regul. 1985, 3, 277–291. [Google Scholar] [CrossRef]
- Livingstone, J.R.; Maruo, T.; Yoshida, I.; Tarui, Y.; Hirooka, K.; Yamamoto, Y.; Tsutui, N.; Hirasawa, E. Purification and properties of betaine aldehyde dehydrogenase from Avena sativa. J. Plant Res. 2003, 116, 133–140. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef]
- Yang, R.; Feng, L.; Wang, S.; Yu, N.; Gu, Z. Accumulation of γ-aminobutyric acid in soybean by hypoxia germination and freeze–thawing incubation. J. Sci. Food Agric. 2016, 96, 2090–2096. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Sartawe, F.A.; Qaryouti, M.M. Characterization of γ-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J. Plant Physiol. 2013, 170, 1003–1009. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Al-Share, A.T. Characterization of the γ-aminobutyric acid shunt pathway and oxidative damage in Arabidopsis thaliana pop2 mutants under various abiotic stresses. Biol. Plantarum. 2016, 60, 132–138. [Google Scholar] [CrossRef]
- Youn, Y.S.; Park, J.K.; Jang, H.D.; Rhee, Y.W. Sequential hydration with anaerobic and heat treatment increases GABA (γ-aminobutyric acid) content in wheat. Food Chem. 2011, 129, 1631–1635. [Google Scholar] [CrossRef]
- Kramer, D.; Breitenstein, B.; Kleinwächter, M.; Selmar, D. Stress metabolism in green coffee beans (Coffea arabica L.): Expression of dehydrins and accumulation of GABA during drying. Plant Cell Physiol. 2010, 51, 546. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, M.; Iki, M.; Aoyagi, Y.; Kim, S.J.; Mukasa, Y.; Yokota, S.; Takigawa, S.; Hashimoto, N.; Noda, T.; et al. Time-course study and effects of drying method on concentrations of γ-aminobutyric acid, flavonoids, anthocyanin, and 2′′-hydroxynicotianamine in leaves of buckwheats. J. Agric. Food Chem. 2009, 57, 259–264. [Google Scholar] [CrossRef]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 73–97. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Busch, K.B.; Fromm, H. Plant succinic semialdehyde dehydrogenase. cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiol. 1999, 121, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, N.; Liu, C.; Wang, H.; Li, Y.; Xie, Y.; Ma, F.; Liang, J.; Li, C. Exogenous GABA improves the resistance of apple seedlings to long-term drought stress by enhancing GABA shunt and secondary cell wall biosynthesis. Tree Physiol. 2022, 42, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cauwenberghe, O.; Shelp, B.J. Biochemical characterization of partially purified gaba:pyruvate transaminase from Nicotiana tabacum. Phytochemistry 1999, 52, 575–581. [Google Scholar] [CrossRef]
- Van Cauwenberghe, O.R.; Makhmoudova, A.; Mclean, M.D.; Clark, S.M.; Shelp, B.J. Plant pyruvate-dependent gamma-aminobutyrate transaminase: Identification of an Arabidopsis cDNA and its expression in Escherichia coli. Can. J. Bot. 2002, 80, 933–941. [Google Scholar] [CrossRef]
- Clark, S.M.; Di Leo, R.; Dhanoa, P.K.; Van Cauwenberghe, O.R.; Mullen, R.T.; Shelp, B.J. Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 2009, 60, 1743. [Google Scholar] [CrossRef] [Green Version]
- Koike, S.; Matsukura, C.; Takayama, M.; Asamizu, E.; Ezura, H. Suppression of gamma -aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol. 2013, 54, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.M.; Di Leo, R.; Van Cauwenberghe, O.R.; Mullen, R.T.; Shelp, B.J. Subcellular localization and expression of multiple tomato gamma-aminobutyrate transaminases that utilize both pyruvate and glyoxylate. J. Exp. Bot. 2009, 60, 3255–3267. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Cheng, C.; Fang, W. Effects of the inhibitor of glutamate decarboxylase on the development and GABA accumulation in germinating fava beans under hypoxia-NaCl stress. RSC Adv. 2018, 8, 20456–20461. [Google Scholar] [CrossRef] [Green Version]
- Andriamampandry, C.; Taleb, O.; Viry, S.; Muller, C.; Humbert, J.P.; Gobaille, S.; Aunis, D.; Maitre, M. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB). FASEB J. 2003, 17, 1691–1693. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef]
- Takayama, M.; Koike, S.; Kusano, M.; Matsukura, C.; Saito, K.; Ariizumi, T.; Ezura, H. Tomato glutamate decarboxylase genes SlGAD2 and SlGAD3 play key roles in regulating gamma-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol. 2015, 56, 1533–1545. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Du, C.; Ji, J.; Xie, T.; Chen, W.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Inhibition of α-ketoglutarate dehydrogenase activity affects adventitious root growth in poplar via changes in GABA shunt. Planta 2018, 248, 963–979. [Google Scholar] [CrossRef]
- Chen, W.; Meng, C.; Ji, J.; Li, M.H.; Zhang, X.; Wu, Y.; Xie, T.; Du, C.; Sun, J.; Jiang, Z.; et al. Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions. Tree Physiol. 2020, 40, 1744–1761. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2018, 9, 1945. [Google Scholar] [CrossRef]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis1. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Bai, S.; Ma, J.; Zhang, L.; Shao, F.; Zhang, K.; Yang, Y.; Sun, T.; Huang, J.; Zhou, Y.; et al. The genome of Populus alba x Populus tremula var. glandulosa clone 84K. DNA Res. 2019, 26, 423–431. [Google Scholar] [CrossRef]
- Deewatthanawong, R.; Rowell, P.; Watkins, C.B. γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biol. Technol. 2010, 57, 97–105. [Google Scholar] [CrossRef]
- Breitkreuz, K.E.; Shelp, B.J.; Fischer, W.N.; Schwacke, R.; Rentsch, D. Identification and characterization of GABA, proline and quaternary ammonium compound transporters from Arabidopsis thaliana. FEBS Lett. 1999, 450, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Eskandari, S.; Grallath, S.; Rentsch, D. AtGAT1, a high affinity transporter for gamma-aminobutyric acid in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 7197–7204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dündar, E.; Bush, D.R. BAT1, a bidirectional amino acid transporter in Arabidopsis. Planta 2009, 229, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Snowden, C.J.; Thomas, B.; Baxter, C.J.; Smith, J.A.; Sweetlove, L.J. A tonoplast Glu/Asp/GABA exchanger that affects tomato fruit amino acid composition. Plant J. 2015, 81, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Cao, X.; Shi, S.; Li, S.; Gao, J.; Ma, Y.; Zhao, Q.; Chen, Q. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol. Biochem. 2016, 107, 164–177. [Google Scholar] [CrossRef]
- Duan, Y.; Zhu, X.; Shen, J.; Xing, H.; Zou, Z.; Ma, Y.; Wang, Y.; Fang, W. Genome-wide identification, characterization and expression analysis of the amino acid permease gene family in tea plants (Camellia sinensis). Genomics 2020, 112, 2866–2874. [Google Scholar] [CrossRef]
- Gilliham, M.; Tyerman, S.D. Linking metabolism to membrane signaling: The GABA–malate connection. Trends Plant Sci. 2015, 21, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Tyerman, S.D.; Gilliham, M. Cytosolic GABA inhibits anion transport by wheat ALMT1. New Phytol. 2020, 225, 671–678. [Google Scholar] [CrossRef]
- Bush, D.R. Inhibitors of the proton-sucrose symport. Biochem. Biophys. 1993, 307, 355–360. [Google Scholar] [CrossRef]
- Ortiz-Lopez, A.; Chang, H.; Bush, D.R. Amino acid transporters in plants. Biochim. Biophys. Acta 2000, 1465, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Frezzini, M.; Guidoni, L.; Pascarella, S. Conformational transitions induced by gamma-amino butyrate binding in GabR, a bacterial transcriptional regulator. Sci. Rep. 2019, 9, 19319. [Google Scholar] [CrossRef] [Green Version]
- Batushansky, A.; Kirma, M.; Grillich, N.; Pham, P.A.; Rentsch, D.; Galili, G.; Fernie, A.R.; Fait, A. The transporter GAT1 plays an important role in GABA-mediated carbon-nitrogen interactions in Arabidopsis. Front. Plant Sci. 2015, 6, 785. [Google Scholar] [CrossRef] [Green Version]
- Young, G.B.; Jack, D.L.; Smith, D.W.; Saier, M.H. The amino acidauxinproton symport permease family. Biochim. Biophys. Acta 1999, 1415, 306–322. [Google Scholar] [CrossRef] [Green Version]
- Wipf, D.; Ludewig, U.; Tegeder, M.; Rentsch, D.; Koch, W.; Frommer, W.B. Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem. Sci. 2002, 27, 139–147. [Google Scholar] [CrossRef]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on amino acid transporter functions and on possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef]
- Tian, R.; Yang, Y.; Chen, M. Genome-wide survey of the amino acid transporter gene family in wheat (Triticum aestivum L.): Identification, expression analysis and response to abiotic stress. Int. J. Biol. Macromol. 2020, 162, 1372–1387. [Google Scholar] [CrossRef]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a transporter for proline, glycine betaine, and gamma-amino butyric acid in tomato pollen. Plant Cell 1999, 11, 377–391. [Google Scholar]
- Fischer, W.N.; Loo, D.D.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Rentsch, D.; Wright, E.M.; Frommer, W.B. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J. 2002, 29, 717–731. [Google Scholar] [CrossRef]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.M.; Rentsch, D. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Wolf-Nicolas, F.; Bruno, A.; Doris, R.; Sylvia, K.; Mechthild, T.; Kevin, B.; Wolf, B.F. Amino acid transport in plants. Trends Plant Sci. 1998, 3, 188–195. [Google Scholar]
- Chen, L.; Ortiz-Lopez, A.; Jung, A.; Bush, D.R. ANT1, an aromatic and neutral amino acid transporter in Arabidopsis. Plant Physiol. 2001, 125, 1813–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, U.G.; Endler, A.; Schelbert, S.; Brunner, A.; Schnell, M.; Neuhaus, H.E. Marty-Mazars, D.; Marty, F.; Baginsky, S.; Martinoia, E. Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds. Plant Physiol. 2007, 145, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, S.A.; Serazetdinova, L.; Jones, A.M.; Sanders, D.; Rathjen, J.; Peck, S.C.; Maathuis, F.J. Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana. Wiley-VCH Verlag GMBH 2008, 8, 3536–3547. [Google Scholar] [CrossRef]
- Ma, D.; Lu, P.; Shi, Y. Substrate selectivity of the acid-activated glutamate/γ-aminobutyric acid (GABA) antiporter GadC from Escherichia coli. J. Biol. Chem. 2013, 288, 15148–15153. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.F.; McCarthy, P.; Miller, C. Substrate selectivity in glutamate-dependent acid resistance in enteric bacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 5898–5902. [Google Scholar] [CrossRef] [Green Version]
- Ham, T.H.; Chu, S.H.; Han, S.J.; Ryu, S.N. γ-aminobutyric acid metabolism in plant under environment stressses. Korean J. Crop Sci. 2012, 57, 144–150. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-aminobutyric acid (GABA) signalling in plants. Cell Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef]
- Streeter, J.G.; Thompson, J.F. Anaerobic accumulation of γ-aminobutyric acid and alanine in Radish Leaves (Raphanus sativus L.). Plant Physiol. 1972, 49, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.W.; Higashi, R.M.; Frenkiel, T.A.; Lane, A.N. Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. J. Exp. Bot. 1997, 48, 1655–1666. [Google Scholar]
- Liao, J.; Wu, X.; Xing, Z.; Li, Q.; Duan, Y.; Fang, W.; Zhu, X. γ-Aminobutyric acid (GABA) accumulation in Tea (Camellia sinensis L.) through the GABA shunt and polyamine degradation pathways under anoxia. J. Agric. Food Chem. 2017, 65, 3013–3018. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J. Exp. Bot. 2008, 59, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 92–102. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Liu, X.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high sailnity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009, 181, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Wang, R.; Chen, S.; Zhou, X.; Shen, X.; Deng, L.; Zhu, H.; Shao, J.; Shi, Y.; Dai, S.; Fritz, E.; et al. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol. 2008, 28, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Shi, Z.; Xie, T.; Zhang, X.; Chen, W.; Du, C.; Sun, J.; Yue, J.; Zhao, X.; Jiang, Z.; et al. Responses of GABA shunt coupled with carbon and nitrogen metabolism in poplar under NaCl and CdCl2 stresses. Ecotoxicol. Environ. Saf. 2020, 193, 110322. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of White Clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in White Clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Singh, C.M.; Kumar, B.; Mehandi, S.; Chandra, K. Effect of drought stress in Rice: A review on morphological and physiological characteristics. Trends Biosci. 2012, 5, 261–265. [Google Scholar]
- Bhusal, B.; Neupane, P.; Regmi, R.; Paudel, M.R.; Bigyan, K.C. A review on abiotic stress resistance in Maize (Zea mays L.): Effects, resistance mechanisms and management. J. Biol. Today’s World 2021, 10, 1–3. [Google Scholar]
- Vanani, F.R.; Shabani, L.; Sabzalian, M.R.; Dehghanian, F.; Winner, L. Comparative physiological and proteomic analysis indicates lower shock response to drought stress conditions in a self-pollinating perennial ryegrass. PloS ONE 2020, 15, e0234317. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.D. GABA: A key player in drought stress resistance in plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.; Hakeem, K.R.; Anwar, Y.; Uddin, N. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2019, 29, 1625–1636. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Xu, Z.; Xu, W.; Li, J.; Zhao, N.; Zhou, Y. Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [Green Version]
- Sussmilch, F.C.; Schultz, J.; Hedrich, R.; Roelfsema, M.R.G. Acquiring Control: The evolution of stomatal signalling pathways. Trends Plant Sci. 2019, 24, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Renault, H.; El Amrani, A.; Palanivelu, R.; Updegraff, E.P.; Yu, A.; Renou, J.P.; Preuss, D.; Bouchereau, A.; Deleu, C. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. Corrigendum: GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 8293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kong, L.; Zhu, Y.; Pei, D.; Chen, X.; Wang, Y.; Qi, J.; Song, C.; Yang, S.; Gong, Z. BAK1 plays contrasting roles in regulating abscisic acid-induced stomatal closure and abscisic acid-inhibited primary root growth in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 17. [Google Scholar] [CrossRef]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.P.; Zhang, Y.; Nie, G.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H.; et al. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in White Clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Sanalkumar, K.; Kevin, L.; Vijaya, S.; Emily, B.M. Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ- aminobutyric acid on Perennial ryegrass. J. Am. Soc. Hortic. Sci. 2013, 138, 358–366. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lü, G.; Gao, H. GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants. Metabolites 2023, 13, 347. https://doi.org/10.3390/metabo13030347
Yuan D, Wu X, Gong B, Huo R, Zhao L, Li J, Lü G, Gao H. GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants. Metabolites. 2023; 13(3):347. https://doi.org/10.3390/metabo13030347
Chicago/Turabian StyleYuan, Ding, Xiaolei Wu, Binbin Gong, Ruixiao Huo, Liran Zhao, Jingrui Li, Guiyun Lü, and Hongbo Gao. 2023. "GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants" Metabolites 13, no. 3: 347. https://doi.org/10.3390/metabo13030347
APA StyleYuan, D., Wu, X., Gong, B., Huo, R., Zhao, L., Li, J., Lü, G., & Gao, H. (2023). GABA Metabolism, Transport and Their Roles and Mechanisms in the Regulation of Abiotic Stress (Hypoxia, Salt, Drought) Resistance in Plants. Metabolites, 13(3), 347. https://doi.org/10.3390/metabo13030347