A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
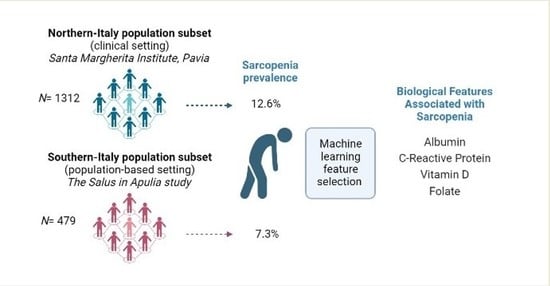
2.1.1. Northern-Italy Population Subset (Santa Margherita Institute, Pavia)
2.1.2. Southern-Italy Population Subset (the Salus in Apulia Study)
2.2. Fluid Biomarker Assessment
2.3. Clinical and Physical Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring Population Ageing: An Analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef]
- The Lancet Healthy Longevity. Care for Ageing Populations Globally. Lancet Healthy Longev. 2021, 2, e180. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Chen, D.; Xie, X.-H.; Zhang, J.-E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a Predictor of Mortality among the Critically Ill in an Intensive Care Unit: A Systematic Review and Meta-Analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in Daily Practice: Assessment and Management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The Role of DXA in Sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Curcio, F.; Ferro, G.; Basile, C.; Liguori, I.; Parrella, P.; Pirozzi, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Tocchetti, C.G.; et al. Biomarkers in Sarcopenia: A Multifactorial Approach. Exp. Gerontol. 2016, 85, 1–8. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Voulgaridou, G.; Kondyli, F.S.; Drakaki, M.; Sianidou, K.; Andrianopoulou, R.; Rodopaios, N.; Pritsa, A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Arai, H.; Assantachai, P.; Akishita, M.; Chew, S.T.H.; Dumlao, L.C.; Duque, G.; Woo, J. Roles of Nutrition in Muscle Health of Community-Dwelling Older Adults: Evidence-Based Expert Consensus from Asian Working Group for Sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 1653–1672. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Huang, J.; Wu, H.; Meng, G.; Zhang, Q.; Liu, L.; Zhang, S.; Wang, X.; Zhang, J.; et al. Serum Vitamin D Status and Circulating Irisin Levels in Older Adults with Sarcopenia. Front. Nutr. 2022, 9, 1051870. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kuchel, G.A. Role of Sarcopenia Definition and Diagnosis in Clinical Care: Moving from Risk Assessment to Mechanism-Guided Interventions. J. Am. Geriatr. Soc. 2020, 68, 1406–1409. [Google Scholar] [CrossRef]
- Lampignano, L.; Bortone, I.; Castellana, F.; Donghia, R.; Guerra, V.; Zupo, R.; de Pergola, G.; di Masi, M.; Giannelli, G.; Lozupone, M.; et al. Impact of Different Operational Definitions of Sarcopenia on Prevalence in a Population-Based Sample: The Salus in Apulia Study. Int. J. Environ. Res. Public Health 2021, 18, 12979. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Donghia, R.; Lampignano, L.; Guerra, V.; de Pergola, G.; Lozupone, M.; Bortone, I.; de Nucci, S.; Tatoli, R.; et al. Liver Frailty and All-Cause Mortality in the Older Participants of the Salus in Apulia Study. Geroscience 2022, 44, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Sardone, R.; Donghia, R.; Castellana, F.; Lampignano, L.; Bortone, I.; Misciagna, G.; de Pergola, G.; Panza, F.; Lozupone, M.; et al. Traditional Dietary Patterns and Risk of Mortality in a Longitudinal Cohort of the Salus in Apulia Study. Nutrients 2020, 12, 1070. [Google Scholar] [CrossRef]
- Sardone, R.; Castellana, F.; Bortone, I.; Lampignano, L.; Zupo, R.; Lozupone, M.; Griseta, C.; Dibello, V.; Seripa, D.; Guerra, V.; et al. Association between Central and Peripheral Age-Related Hearing Loss and Different Frailty Phenotypes in an Older Population in Southern Italy. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 561–571. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010; ISBN 9781139488150. [Google Scholar]
- Grissom, R.J.; Kim, J.J. Effect Sizes for Research: A Broad Practical Approach; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2005; Volume 253. [Google Scholar]
- Shigehara, K.; Kato, Y.; Izumi, K.; Mizokami, A. Relationship between Testosterone and Sarcopenia in Older-Adult Men: A Narrative Review. J. Clin. Med. Res. 2022, 11, 6202. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Zamboni, M.; Rubele, S.; Rossi, A.P. Sarcopenia and Obesity. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Peroni, G.; Faliva, M.A.; Bartolo, A.; Naso, M.; Miccono, A.; Rondanelli, M. Sarcopenia and Sarcopenic Obesity in Comparison: Prevalence, Metabolic Profile, and Key Differences. A Cross-Sectional Study in Italian Hospitalized Elderly. Aging Clin. Exp. Res. 2017, 29, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wong, M.; Leung, J.; Lee, J.; Auyeung, T.W.; Woo, J. Incidence, Reversibility, Risk Factors and the Protective Effect of High Body Mass Index against Sarcopenia in Community-Dwelling Older Chinese Adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 15–28. [Google Scholar] [CrossRef]
- Yoo, M.C.; Won, C.W.; Soh, Y. Association of High Body Mass Index, Waist Circumference, and Body Fat Percentage with Sarcopenia in Older Women. BMC Geriatr. 2022, 22, 937. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. Res. 2019, 8, 775. [Google Scholar] [CrossRef]
- Pergola, G.D.; de Pergola, G.; Zupo, R.; Lampignano, L.; Paradiso, S.; Murro, I.; Bartolomeo, N.; Cecere, A.; Ciccone, M.M.; Giannelli, G.; et al. Effects of a Low Carb Diet and Whey Proteins on Anthropometric, Hematochemical and Cardiovascular Parameters in Subjects with Obesity. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1719–1725. [Google Scholar] [CrossRef]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower Serum Albumin Concentration and Change in Muscle Mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [PubMed]
- Cantin, A.M.; Paquette, B.; Richter, M.; Larivée, P. Albumin-Mediated Regulation of Cellular Glutathione and Nuclear Factor Kappa B Activation. Am. J. Respir. Crit. Care Med. 2000, 162, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.K.H. Serum Folate Predicts Muscle Strength: A Pilot Cross-Sectional Study of the Association between Serum Vitamin Levels and Muscle Strength and Gait Measures in Patients >65 Years Old with Diabetes Mellitus in a Primary Care Setting. Nutr. J. 2016, 15, 89. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; de Nucci, S.; Sila, A.; Aresta, S.; Buscemi, C.; Randazzo, C.; Buscemi, S.; Triggiani, V.; de Pergola, G.; et al. Role of Dietary Carotenoids in Frailty Syndrome: A Systematic Review. Biomedicines 2022, 10, 632. [Google Scholar] [CrossRef]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Pilleron, S.; Féart, C.; García García, F.J.; Bandinelli, S.; Gomez-Cabrero, D.; Rodriguez-Mañas, L.; Grune, T.; et al. Associations of Fat-Soluble Micronutrients and Redox Biomarkers with Frailty Status in the FRAILOMIC Initiative. J. Cachexia Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef]
- Miller, A.L. The Methylation, Neurotransmitter, and Antioxidant Connections between Folate and Depression. Altern. Med. Rev. 2008, 13, 216–226. [Google Scholar]
- Wang, J.; Wang, X.; Gu, Y.; Liu, M.; Chi, V.T.Q.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; et al. Vitamin D Is Related to Handgrip Strength in Adult Men Aged 50 Years and over: A Population Study from the TCLSIH Cohort Study. Clin. Endocrinol. 2019, 90, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.P.; Visser, M.; Deeg, D.J.H.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D Status Predicts Physical Performance and Its Decline in Older Persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The Effect of Vitamin D Plus Protein Supplementation on Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.; Monteferrario, F.; Peroni, G.; Repaci, E.; Allieri, F.; Perna, S. Novel Insights on Nutrient Management of Sarcopenia in Elderly. BioMed Res. Int. 2015, 2015, 524948. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory Markers and Physical Performance in Older Persons: The InCHIANTI Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M242–M248. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and Sarcopenia: A Systematic Review and Meta-Analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 Level and the Development of Disability in Older Persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory Markers and Loss of Muscle Mass (sarcopenia) and Strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-F.; Yeh, Y.-L.; Chang, H.-Y.; Tsai, S.-H.; Wang, J.-Y. Prevalence and Risk Factors of Sarcopenia among Older Adults Aged ≥65 Years Admitted to Daycare Centers of Taiwan: Using AWGS 2019 Guidelines. Int. J. Environ. Res. Public Health 2021, 18, 8299. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Bibilonic, M.; Tur, J.A. Anthropometry, Body Composition and Resting Energy Expenditure in Human. Nutrients 2019, 11, 1891. [Google Scholar]
- Kang, Y.-J.; Yoo, J.-I.; Ha, Y.-C. Sarcopenia Feature Selection and Risk Prediction Using Machine Learning: A Cross-Sectional Study. Medicine 2019, 98, e17699. [Google Scholar] [CrossRef] [PubMed]


| Clinical-Based Subset (Pavia) | Population-Based Subset (Apulia) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Wilcoxon’s ES | |
| Prop. (%) | 1312 (73.30) | 479 (26.70) | |||
| Age (years) | 79.79 ± 7.18 | 80 (10) | 74.81 ± 5.67 | 74.09 (7.58) | 0.32 (0.28 to 0.36) |
| Sex | |||||
| Female | 949 (72.30) | 255 (53.20) | 19.10 (14.01 to 24.18) | ||
| Male | 363 (27.70) | 224 (46.80) | |||
| Sarcopenia | 165 (12.60) | 35 (7.30) | −5.27 (−8.21 to −2.33) | ||
| Albumin (g/dL) | 3.67 ± 0.51 | 3.74 (0.67) | 4.08 ± 0.42 | 4.05 (0.4) | 0.37 (0.34 to 0.41) |
| AST (U/L) | 18.65 ± 17.7 | 14 (10) | 20.39 ± 9.33 | 19 (8) | 0.26 (0.22 to 0.30) |
| BMI (kg/m2) | 25.57 ± 5.88 | 24.7 (6.7) | 29.75 ± 4.94 | 29.32 (6.08) | 0.26 (0.22 to 0.30) |
| CRP (mg/dL) | 1.25 ± 2.63 | 0.35 (0.84) | 0.43 ± 0.62 | 0.43 (0.33) | 0.02 (−0.01 to 0.05) |
| FBG (mg/dL) | 108.19 ± 41.06 | 96 (33) | 102.68 ± 25.31 | 96 (21) | 0.01 (−0.03 to 0.03) |
| FFM arms (kg) | 4.12 ± 1.38 | 3.86 (1.70) | 4.71 ± 1.354 | 4.51 (2.15) | 0.20 (0.16 to 0.24) |
| FFM legs (kg) | 13.419 ± 3.21 | 12.98 (4.33) | 13.24 ± 3.02 | 13.14 (4.71) | 0.02 (−0.03 to 0.04) |
| Folate (ng/mL) | 9.13 ± 9.19 | 5.9 (7.82) | 8.98 ± 5.95 | 7.6 (5.3) | 0.11 (0.08 to 0.16) |
| FT3 (pmol/L) | 2.32 ± 0.53 | 2.3 (0.64) | 3.32 ± 0.39 | 3.32 (0.46) | 0.67 (0.65 to 0.70) |
| FT4 (pmol/L) | 5.76 ± 5.74 | 1.46 (9.78) | 0.97 ± 0.66 | 0.9 (0.19) | 0.54 (0.51 to 0.58) |
| GGT (U/L) | 32.98 ± 42.09 | 20 (18) | 19.44 ± 16.61 | 15 (9) | 0.21 (0.17 to 0.26) |
| HGS (kg) | 18.68 ± 7.74 | 18 (9.33) | 23.13 ± 8.28 | 22 (11.67) | 0.24 (0.20 to 0.29) |
| HDL Cholesterol (mg/dL) | 47.96 ± 17.65 | 47 (21) | 52.41 ± 14.02 | 52 (17) | 0.12 (0.08 to 0.17) |
| Height (cm) | 156.79 ± 9.62 | 155 (13) | 157.45 ± 9.03 | 157 (13) | 0.04 (−0.01 to 0.09) |
| Haemoglobin (g/dL) | 12.35 ± 1.72 | 12.4 (2.2) | 13.68 ± 1.44 | 13.7 (1.8) | 0.35 (0.31 to 0.39) |
| LDL Cholesterol (mg/dL) | 111.76 ± 37.32 | 107.9 (48.85) | 104.9 ± 32.66 | 104 (49) | 0.07 (0.20 to 0.12) |
| Neck BMD | 0.74 ± 0.18 | 0.73 (0.2) | 0.77 ± 0.58 | 0.7 (0.17) | 0.04 (0.01 to 0.09) |
| Platelets (103 cells/mm3) | 247.02 ± 103.99 | 238.6 (103.72) | 230.55 ± 71.2 | 226 (75.5) | 0.07 (0.03 to 0.12) |
| RBC (106 cells/mm3) | 4.18 ± 0.65 | 4.17 (0.69) | 4.66 ± 0.55 | 4.66 (0.62) | 0.38 (0.34 to 0.42) |
| SMI (kg/m2) | 7.08 ± 1.37 | 7 (1.72) | 7.17 ± 1.18 | 7.1 (1.75) | 0.03 (−0.01 to 0.08) |
| SPPB score | 6.26 ± 3.04 | 6 (5) | 8.27 ± 2.86 | 9 (5) | 0.28 (0.25 to 0.33) |
| Total Cholesterol (mg/dL) | 185.68 ± 41.34 | 184.5 (58) | 180.14 ± 37.31 | 179 (50.5) | 0.05 (0.01 to 0.10) |
| Triglycerides (mg/dL) | 132.2 ± 80.08 | 111 (75.25) | 113.56 ± 75.43 | 93 (63.5) | 0.13 (0.09 to 0.18) |
| TSH (µU/mL) | 2.13 ± 2.28 | 1.5 (1.61) | 1.87 ± 1.76 | 1.55 (1.29) | 0.01 (−0.03 to 0.03) |
| Vitamin D (nmol/L) | 13.29 ± 10.77 | 10.2 (10.2) | 27.98 ± 12.69 | 26.4 (12.7) | 0.54 (0.51 to 0.58) |
| WBC (103 cells/mm3) | 7.01 ± 3.14 | 6.54 (2.41) | 6.36 ± 1.81 | 6.09 (2.13) | 0.10 (0.06 to 0.15) |
| Whole Body Fat (kg) | 22.60 ± 10.46 | 21.30 (13.26) | 30.04 ± 8.943 | 28.68 (11.84) | 0.34 (0.30 to 0.38) |
| Whole Body Lean Mass (kg) | 38.84 ± 7.928 | 37.29 (10.52) | 42.77 ± 8.503 | 41.61 (13.56) | 0.20 (0.16 to 0.25) |
| Whole Body Mass (kg) | 63.43 ± 15.59 | 61.70 (20.01) | 72.82 ± 13.93 | 72.34 (18.93) | 0.28 (0.25 to 0.33) |
| Weight (kg) | 62.85 ± 15.53 | 60.55 (19.2) | 73.86 ± 14.31 | 72.6 (18.7) | 0.33 (0.30 to 0.37) |
| Without Sarcopenia | With Sarcopenia | ||||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Wilcoxon’s Effect Size | |
| Prop. (%) | 1591 (88.80) | 200 (11.20) | |||
| Age (years) | 78.04 ± 7.1 | 78 (10.67) | 81.79 ± 6.76 | 82.1 (10) | 0.16 (0.12 to 0.21) |
| Sex | |||||
| Female | 1113 (70.00) | 91 (45.50) | 24.46 (17.20 to 31.72) | ||
| Male | 478 (30.00) | 109 (54.50) | |||
| Population setting | |||||
| Pavia subset | 1147 (72.10) | 165 (82.50) | −10.41 (−16.12 to −4.70) | ||
| Apulia subset | 444 (27.90) | 35 (17.50) | |||
| Albumin (g/dL) | 3.81 ± 0.5 | 3.87 (0.57) | 3.53 ± 0.62 | 3.56 (0.9) | 0.15 (0.12 to 0.22) |
| AST (U/L) | 18.98 ± 15.17 | 16 (11) | 20.15 ± 20.96 | 14 (11) | 0.03 (−0.01 to 0.08) |
| BMI (kg/m2) | 27.35 ± 5.83 | 26.64 (7) | 21.47 ± 3.92 | 21.3 (5.12) | 0.33 (0.30 to 0.37) |
| CRP (mg/dL) | 0.9 ± 2.02 | 0.34 (0.44) | 2.1 ± 3.76 | 0.5 (1.92) | 0.11 (0.06 to 0.16) |
| FBG (mg/dL) | 106.47 ± 36.6 | 96 (29) | 108.74 ± 44.6 | 93.5 (35) | 0.02 (−0.02 to 0.05) |
| FFM arms (kg) | 4.38 ± 1.38 | 4.05 (1.91) | 3.45 ± 1.24 | 3.292 (2.00) | 0.19 (0.15 to 0.25) |
| FFM legs (kg) | 13.70 ± 3.08 | 13.31 (4.45) | 10.73 ± 2.45 | 10.52 (3.53) | 0.29 (0.25 to 0.33) |
| Folate (ng/mL) | 8.86 ± 7.9 | 6.4 (6.8) | 10.91 ± 11.75 | 6.85 (10.85) | 0.03 (−0.01 to 0.08) |
| FT3 (pmol/L) | 2.62 ± 0.66 | 2.55 (0.89) | 2.32 ± 0.7 | 2.32 (0.74) | 0.13 (0.09 to 0.18) |
| FT4 (pmol/L) | 4.64 ± 5.42 | 1.21 (9.27) | 3.14 ± 4.61 | 1.18 (0.52) | 0.01 (−0.02 to 0.04) |
| GGT (U/L) | 28.72 ± 35.73 | 18 (15) | 34.5 ± 49.29 | 21 (17) | 0.04 (−0.01 to 0.09) |
| HGS (kg) | 20.45 ± 8.19 | 19 (10.17) | 15.26 ± 5.92 | 14 (9) | 0.20 (0.16 to 0.24) |
| HDL Cholesterol (mg/dL) | 49.66 ± 16.71 | 49 (21) | 45.11 ± 17.6 | 44 (21) | 0.08 (0.04 to 0.13) |
| Height (cm) | 156.79 ± 9.38 | 156 (13) | 158.33 ± 10.05 | 158 (15) | 0.04 (−0.01 to 0.08) |
| Haemoglobin (g/dL) | 12.76 ± 1.74 | 12.9 (2.3) | 12.28 ± 1.8 | 12.3 (2.35) | 0.09 (0.05 to 0.14) |
| LDL Cholesterol (mg/dL) | 110.64 ± 36.16 | 107.4 (48.3) | 104.2 ± 36.59 | 100.2 (52.5) | 0.05 (0.01 to 0.10) |
| Neck BMD | 0.76 ± 0.35 | 0.72 (0.19) | 0.71 ± 0.22 | 0.69 (0.24) | 0.06 (0.02 to 0.11) |
| Platelets (103 cells/mm3) | 242.83 ± 95.19 | 237 (91.45) | 240.9 ± 107.23 | 225.9 (103.33) | 0.01 (−0.03 to 0.04) |
| RBC (106 cells/mm3) | 4.32 ± 0.66 | 4.32 (0.75) | 4.17 ± 0.63 | 4.18 (0.73) | 0.07 (0.03 to 0.12) |
| SMI (kg/m2) | 7.29 ± 1.24 | 7.19 (1.73) | 5.59 ± 0.86 | 5.44 (1.32) | 0.40 (0.37 to 0.44) |
| SPPB score | 6.94 ± 3.1 | 7 (4) | 5.62 ± 3.03 | 6 (5) | 0.13 (0.09 to 0.18) |
| Total Cholesterol (mg/dL) | 185.45 ± 39.86 | 184 (55) | 174.24 ± 43.02 | 169 (64) | 0.09 (0.05 to 0.14) |
| Triglycerides (mg/dL) | 127.36 ± 79.02 | 106 (74) | 126.07 ± 81.52 | 100.5 (63) | 0.13 (−0.03 to 0.04) |
| TSH (µU/mL) | 2.1 ± 2.23 | 1.54 (1.55) | 1.74 ± 1.37 | 1.42 (1.48) | 0.04 (0.01 to 0.08) |
| Vitamin D (nmol/L) | 17.36 ± 13.01 | 13.9 (16.85) | 16.13 ± 13.31 | 10.9 (12.43) | 0.04 (0.01 to 0.09) |
| WBC (103 cells/mm3) | 6.76 ± 2.9 | 6.36 (2.3) | 7.46 ± 2.41 | 6.98 (2.83) | 0.10 (0.06 to 0.15) |
| Whole Body Fat (kg) | 25.55 ± 10.50 | 24.49 (14.07) | 16.94 ± 7.91 | 16.58 (10.77) | 0.26 (0.23 to 0.31) |
| Whole Body Lean Mass (kg) | 40.47 ± 82.44 | 38.75 (12.35) | 35.28 ± 6.91 | 34.64 (11.30) | 0.19 (0.15 to 0.24) |
| Whole Body Mass (kg) | 67.47 ± 15.46 | 66.28 (20.93) | 53.78 ± 12.05 | 53.64 (16.62) | 0.27 (0.24 to 0.32) |
| Weight (kg) | 67.25 ± 15.83 | 65.4 (21) | 54.23 ± 11.87 | 54.3 (17.23) | 0.26 (0.22 to 0.30) |
| Prediction | |||
|---|---|---|---|
| Without | With | ||
| Test dataset (Salus) | Without | 444 (94.50) | -- |
| With | 26 (5.50) | 9 (100.00) | |
| Accuracy (CI 95%) | |||
| 94.57 (92.15 to 96.42) | |||
| Sensitivity | |||
| 1.00 | |||
| Specificity | |||
| 0.94 | |||
| OR | Stand. Err. | CI 95% | p-Value | |
|---|---|---|---|---|
| (Intercept) | 0.013 | 1.61 | 0.001 to 0.301 | <0.01 |
| Age (years) | 1.054 | 0.01 | 1.024 to 1.085 | <0.01 |
| Sex (Male) | 4.384 | 0.18 | 3.027 to 6.351 | <0.01 |
| SPPB score | 0.906 | 0.03 | 0.847 to 0.969 | <0.01 |
| WBC (103 cells/mm3) | 1.051 | 0.01 | 0.998 to 1.108 | 0.07 |
| RBC (106 cells/mm3) | 1.200 | 0.16 | 0.860 to 1.674 | 0.28 |
| Platelets (103 cells/mm3) | 1.001 | 0.01 | 0.999 to 1.002 | 0.76 |
| FBG (mg/dL) | 0.999 | 0.01 | 0.995 to 1.003 | 0.57 |
| Triglycerides (mg/dL) | 1.001 | 0.01 | 0.989 to 1.013 | 0.88 |
| Total Cholesterol (mg/dL) | 1.004 | 0.01 | 0.950 to 1.062 | 0.87 |
| HDL Cholesterol (mg/dL) | 1.001 | 0.01 | 0.943 to 1.062 | 0.98 |
| LDL Cholesterol (mg/dL) | 0.997 | 0.01 | 0.943 to 1.053 | 0.90 |
| TSH (µU/mL) | 0.871 | 0.01 | 0.784 to 0.969 | 0.01 |
| FT3 (pmol/L) | 0.555 | 0.17 | 0.393 to 0.783 | <0.01 |
| FT4 (pmol/L) | 0.921 | 0.01 | 0.887 to 0.956 | <0.01 |
| CRP (mg/dL) | 1.065 | 0.01 | 0.999 to 1.135 | 0.06 |
| Folate (ng/mL) | 1.022 | 0.01 | 1.005 to 1.038 | <0.01 |
| Vitamin D (nmol/L) | 1.015 | 0.01 | 1.002 to 1.028 | 0.01 |
| Haemoglobin (g/dL) | 1.007 | 0.01 | 0.873 to 1.16 | 0.92 |
| GGT (U/L) | 0.999 | 0.01 | 0.995 to 1.004 | 0.73 |
| AST (U/L) | 0.999 | 0.01 | 0.989 to 1.011 | 0.99 |
| Albumin (g/dL) | 0.562 | 0.19 | 0.386 to 0.818 | <0.01 |
| Reference | |||
|---|---|---|---|
| No | Yes | ||
| Prediction | No | 1581 | 171 |
| Yes | 10 | 29 | |
| Accuracy | 89.89 | ||
| Sensitivity | 14.50 | ||
| Specificity | 99.37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupo, R.; Moroni, A.; Castellana, F.; Gasparri, C.; Catino, F.; Lampignano, L.; Perna, S.; Clodoveo, M.L.; Sardone, R.; Rondanelli, M. A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations. Metabolites 2023, 13, 565. https://doi.org/10.3390/metabo13040565
Zupo R, Moroni A, Castellana F, Gasparri C, Catino F, Lampignano L, Perna S, Clodoveo ML, Sardone R, Rondanelli M. A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations. Metabolites. 2023; 13(4):565. https://doi.org/10.3390/metabo13040565
Chicago/Turabian StyleZupo, Roberta, Alessia Moroni, Fabio Castellana, Clara Gasparri, Feliciana Catino, Luisa Lampignano, Simone Perna, Maria Lisa Clodoveo, Rodolfo Sardone, and Mariangela Rondanelli. 2023. "A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations" Metabolites 13, no. 4: 565. https://doi.org/10.3390/metabo13040565
APA StyleZupo, R., Moroni, A., Castellana, F., Gasparri, C., Catino, F., Lampignano, L., Perna, S., Clodoveo, M. L., Sardone, R., & Rondanelli, M. (2023). A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations. Metabolites, 13(4), 565. https://doi.org/10.3390/metabo13040565