Bioactive Lichen Secondary Metabolites and Their Presence in Species from Chile
Abstract
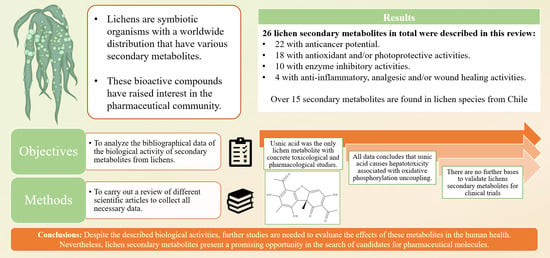
:1. Introduction
2. Materials and Methods
3. Lichen Species of Pharmaceutical Interest
3.1. Lichen Species from the Northern Hemisphere of Pharmaceutical Interest
3.2. Chilean Lichen Species of Pharmaceutical Interest
3.3. Lichen Secondary Metabolites
3.4. Usnic Acid
3.5. Atranorin
3.6. Lobaric Acid
3.7. Gyrophoric Acid
3.8. Fumarprotocetraric Acid
3.9. Protolichesterinic Acid
3.10. Physodic Acid
3.11. Diffractaic Acid
3.12. Salazinic Acid
3.13. Other Secondary Metabolites
4. Pharmacological and Toxicological Considerations
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Müller, K. Pharmaceutically Relevant Metabolites from Lichens. Appl. Microbiol. Biotechnol. 2001, 56, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Seaward, M.R.D. Environmental Role of Lichens. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 274–298. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current Results on Biological Activities of Lichen Secondary Metabolites: A Review. Z. Naturforsch. C J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Sancho, L.G.; De la Torre, R.; Horneck, G.; Ascaso, C.; De Los Rios, A.; Pintado, A.; Wierzchos, J.; Schuster, M. Lichens Survive in Space: Results from the 2005 Lichens Experiment. Astrobiology 2007, 7, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.J. The Growth of Lichens with Special Reference to the Modelling of Circular Thalli. Lichenologist 1981, 13, 265–287. [Google Scholar] [CrossRef]
- Zakeri, Z.; Junne, S.; Jäger, F.; Dostert, M.; Otte, V.; Neubauer, P. Lichen Cell Factories: Methods for the Isolation of Photobiont and Mycobiont Partners for Defined Pure and Co-Cultivation. Microb. Cell Fact. 2022, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar] [CrossRef]
- Boustie, J.; Grube, M. Lichens—A Promising Source of Bioactive Secondary Metabolites. Plant Genet Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen Secondary Metabolites Are Responsible for Induction of Apoptosis in HT-29 and A2780 Human Cancer Cell Lines. Toxicol. Vitr. 2012, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Vargas Castillo, R.; Sandoval Leiva, P. Lista Sistemática de Los Hongos Liquenizados y Liquenícolas Presentes en Chile; Version 1.6; Checklist Dataset; Universidad Metropolitana de Ciencias de la Educación: Santiago, Chile, 2020. [Google Scholar] [CrossRef]
- Crawford, S.D. Lichens Used in Traditional Medicine. In Lichen Secondary Metabolites; Ranković, B., Ed.; Springer: Cham, Switzerland, 2019; pp. 31–97. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 Classification of Lichenized Fungi in the Ascomycota and Basidiomycota—Approaching One Thousand Genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Gómez-Serranillos, M.P.; Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A. Parmeliaceae Family: Phytochemistry, Pharmacological Potential and Phylogenetic Features. RSC Adv. 2014, 4, 59017–59047. [Google Scholar] [CrossRef]
- Xu, M.; Heidmarsson, S.; Olafsdottir, E.S.; Buonfiglio, R.; Kogej, T.; Omarsdottir, S. Secondary Metabolites from Cetrarioid Lichens: Chemotaxonomy, Biological Activities and Pharmaceutical Potential. Phytomedicine 2016, 23, 441–459. [Google Scholar] [CrossRef]
- Ristic, S.; Rankovic, B.; Kosanić, M.; Stamenkovic, S.; Stanojković, T.; Sovrlić, M.; Manojlović, N. Biopharmaceutical Potential of Two Ramalina Lichens and Their Metabolites. Curr. Pharm. Biotechnol. 2016, 17, 651–658. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia Lichens and Their Major Metabolites as Possible Natural Antioxidant, Antimicrobial and Anticancer Agents. LWT 2014, 1, 518–525. [Google Scholar] [CrossRef]
- Grujičić, D.; Stošić, I.; Kosanić, M.; Stanojković, T.; Ranković, B.; Milošević-Djordjević, O. Evaluation of in vitro Antioxidant, Antimicrobial, Genotoxic and Anticancer Activities of Lichen Cetraria Islandica. Cytotechnology 2014, 66, 803. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Küpeli Akkol, E.; Süntar, I.; Keleş, H.; Yildiz, S.; Çaliş, I. Biological Activities of Pseudevernia furfuracea (L.) Zopf Extracts and Isolation of the Active Compounds. J. Ethnopharmacol. 2012, 144, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Divya Reddy, S.; Siva, B.; Kumar, K.; Phani Babu, V.S.; Sravanthi, V.; Boustie, J.; Lakshma Nayak, V.; Tiwari, A.K.; Rao, C.H.V.; Sridhar, B.; et al. Comprehensive Analysis of Secondary Metabolites in Usnea longissima (Lichenized Ascomycetes, Parmeliaceae) Using UPLC-ESI-QTOF-MS/MS and Pro-Apoptotic Activity of Barbatic Acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mosbach, E.W. Botánica Indigena de Chile; Andres Bello: Santiago, Chile, 1992. [Google Scholar]
- Gandhi, A.D.; Sathiyaraj, S.; Suriyakala, G.; Saranya, S.; Baskaran, T.N.; Ravindran, B.; Babujanarthanam, R. Lichens in Genus Parmelia: An Overview and Their Application. Curr. Pharm. Biotechnol. 2020, 21, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Chamy, M.C.; Gambaro, V.; Garbarino, J.A.; Quilhot, W. Studies on Chilean Lichens, VII. The Phenolic Constituents of Protusnea malacea. J. Nat. Prod. 2004, 48, 307–309. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Chamy, M.C.; Gambaro, V.; Quilhot, W.; Naranjc, O.; Bolt, E. Studies on Chilean Lichens, X. The Phenolic Constituents of Protousnea magellanica. J. Nat. Prod. 2004, 50, 745–747. [Google Scholar] [CrossRef]
- Garbarino, J. Estudio de Líquenes Chilenos. XIX. Investigaciones de Metabolitos Secundarios En Líquenes Antárticos. 1993. Available online: gaia.umag.cl/handle/20.500.11894/762 (accessed on 14 June 2023).
- Piovano, M.; Garrido, M.I.; Gambaro, V.; Garbarino, J.A.; Quilhot, W. Studies on Chilean Lichens, VIII. Depsidones from Psoroma Species. J. Nat. Prod. 2004, 48, 854–855. [Google Scholar] [CrossRef]
- Chamy, M.; Fiedler, P.; Piovano, M.; Quilhot, W.; Garbarino, J.A. Studies on Chilean Lichens. XXI. Secondary Metabolites from the Antarctic Species Hypogymnia lugubris. In Serie Científica; Instituto Antártico Chileno: Punta Arenas, Chile, 1993; pp. 81–85. [Google Scholar]
- Quilhot, W.; DlDYK, B.; Gambaro, V.; Garbarino, J.A. Studies on Chilean Lichens, VI. Depsidones from Erioderma chilense. J. Nat. Prod. 1983, 46, 942–943. [Google Scholar] [CrossRef]
- Quilhot, W.; Garbarino, J.; Piovano, M.; Chamy, M. Studies on Chilean Lichens. XI Secondary Metabolites from Antarctic Lichens. Ser. Cient. INACH 1989, 39, 75–89. [Google Scholar]
- Quilhot, W.; Piovano, M.; Arancibia, H.; Garbarino, J.A.; Gambaro, V. Studies on Chilean Lichens, XII. Chemotaxonomy of the Genus Psoroma. J. Nat. Prod. 2004, 52, 191–192. [Google Scholar] [CrossRef]
- Piovano, M.; Garbarino, J.; Chamy, M.; Zúñiga, V. Studies on Chilean Lichens. XVI. Advances in the Chemistry of Secondary Metabolites from Antarctic Lichens. Ser. Cient. INACH 1991, 41, 79–90. [Google Scholar]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic Activity and Antioxidant Capacity of Purified Lichen Metabolites: An in vitro Study. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential Anticancer Activity of Lichen Secondary Metabolite Physodic Acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- Yang, Y.; Park, S.Y.; Nguyen, T.T.; Yu, Y.H.; Nguyen, T.; Sun, E.G.; Udeni, J.; Jeong, M.H.; Pereira, I.; Moon, C.; et al. Lichen Secondary Metabolite, Physciosporin, Inhibits Lung Cancer Cell Motility. PLoS ONE 2015, 10, e0137889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nguyen, T.T.; Pereira, I.; Hur, J.S.; Kim, H. Lichen Secondary Metabolite Physciosporin Decreases the Stemness Potential of Colorectal Cancer Cells. Biomolecules 2019, 9, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Bhosle, S.R.; Yu, Y.H.; Park, S.Y.; Zhou, R.; Taş, I.; Gamage, C.D.B.; Kim, K.K.; Pereira, I.; Hur, J.S.; et al. Tumidulin, a Lichen Secondary Metabolite, Decreases the Stemness Potential of Colorectal Cancer Cells. Molecules 2018, 23, 2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingólfsdóttr, K. Usnic Acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Dinçsoy, A.B.; Cansaran Duman, D. Changes in Apoptosis-Related Gene Expression Profiles in Cancer Cell Lines Exposed to Usnic Acid Lichen Secondary Metabolite. Turk. J. Biol. 2017, 41, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Yurdacan, B.; Egeli, U.; Eskiler, G.G.; Eryilmaz, I.E.; Cecener, G.; Tunca, B. The Role of Usnic Acid-Induced Apoptosis and Autophagy in Hepatocellular Carcinoma. Hum. Exp. Toxicol. 2019, 38, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zhang, X.; Zhou, B.; Zhang, C.; Tu, J.; Chen, X.; Wang, J.; Gao, H.; Qin, G.; Pan, W. Usnic Acid Induces Cycle Arrest, Apoptosis, and Autophagy in Gastric Cancer Cells in vitro and in vivo. Med. Sci. Monit. 2018, 24, 556–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Nambiar, D.; Kale, R.K.; Singh, R.P. Usnic Acid Inhibits Growth and Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma A549 Cells. Nutr. Cancer 2013, 65 (Suppl. S1), 36–43. [Google Scholar] [CrossRef]
- Zuo, S.T.; Wang, L.P.; Zhang, Y.; Zhao, D.N.; Li, Q.S.; Shao, D.; Fang, X.D. Usnic Acid Induces Apoptosis via an ROS-Dependent Mitochondrial Pathway in Human Breast Cancer Cells in vitro and in vivo. RSC Adv. 2014, 5, 153–162. [Google Scholar] [CrossRef]
- Behera, B.C.; Mahadik, N.; Morey, M. Antioxidative and Cardiovascular-Protective Activities of Metabolite Usnic Acid and Psoromic Acid Produced by Lichen Species Usnea complanata under Submerged Fermentation. Pharm. Biol. 2012, 50, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Cetin Cakmak, K.; Gülçin, İ. Anticholinergic and Antioxidant Activities of Usnic Acid-an Activity-Structure Insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective Effects of Lichen Metabolites Evernic and Usnic Acids against Redox Impairment-Mediated Cytotoxicity in Central Nervous System-like Cells. Food Chem. Toxicol. 2017, 105, 262–277. [Google Scholar] [CrossRef]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; dos Santos, J.P.A.; da Rocha, R.F.; Pasquali, M.A.d.B.; Rybarczyk-Filho, J.L.; Araújo, A.A.S.; Moreira, J.C.F.; Gelain, D.P. Redox Characterization of Usnic Acid and Its Cytotoxic Effect on Human Neuron-like Cells (SH-SY5Y). Toxicol. Vitr. 2012, 26, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Legouin, B.; Lohézic-Le Dévéhat, F.; Ferron, S.; Rouaud, I.; Le Pogam, P.; Cornevin, L.; Bertrand, M.; Boustie, J. Specialized Metabolites of the Lichen Vulpicida pinastri Act as Photoprotective Agents. Molecules 2017, 22, 1162. [Google Scholar] [CrossRef]
- Su, Z.Q.; Mo, Z.Z.; Liao, J.B.; Feng, X.X.; Liang, Y.Z.; Zhang, X.; Liu, Y.H.; Chen, X.Y.; Chen, Z.W.; Su, Z.R.; et al. Usnic Acid Protects LPS-Induced Acute Lung Injury in Mice through Attenuating Inflammatory Responses and Oxidative Stress. Int. Immunopharmacol. 2014, 22, 371–378. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, G.; Tao, J.; Ruan, J. Anti-Inflammatory Effects and Mechanisms of Usnic Acid. J. Wuhan Univ. Technol. Mater. 2011, 26, 955–959. [Google Scholar] [CrossRef]
- Sujatha, D.; Hepsiba Rani, C.; Begum, S.; Sampathi, S.; Shah, S. In Silico, In Vitro and In Vivo Anti-Inflammatory and Analgesic Activity of Usnic Acid. In Advances in Computational and Bio-Engineering: Proceeding of the International Conference on Computational and Bio Engineering; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 249–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Li, Y.; Bai, H.; Ma, T.; Song, X.; Zhao, J.; Gao, L. The Effects of Sodium Usnic Acid by Topical Application on Skin Wound Healing in Rats. Biomed. Pharmacother. 2018, 97, 587–593. [Google Scholar] [CrossRef]
- Verma, N.; Behera, B.C.; Sharma, B.O. Glucosidase Inhibitory and Radical Scavenging Properties of Lichen Metabolites Salazinic Acid, Sekikaic Acid and Usnic Acid. HJBC 2012, 40, 7–21. [Google Scholar] [CrossRef]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini. Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable Responses of Different Human Cancer Cells to the Lichen Compounds Parietin, Atranorin, Usnic Acid and Gyrophoric Acid. Toxicol. Vitr. 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Kim, S.; Kim, J.H.; Youn, U.J.; Suh, S.S. The Comprehensive Roles of ATRANORIN, A Secondary Metabolite from the Antarctic Lichen Stereocaulon caespitosum, in HCC Tumorigenesis. Molecules 2019, 24, 1414. [Google Scholar] [CrossRef] [Green Version]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Węgrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyż, J.; et al. Usnic Acid and Atranorin Exert Selective Cytostatic and Anti-Invasive Effects on Human Prostate and Melanoma Cancer Cells. Toxicol. Vitr. 2017, 40, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, Y.; Park, S.Y.; Nguyen, T.T.; Seo, Y.W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.S.; Kim, H. The Lichen Secondary Metabolite Atranorin Suppresses Lung Cancer Cell Motility and Tumorigenesis. Sci. Rep. 2017, 7, 8136. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.G.D.; dos Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; de Bittencourt Pasquali, M.A.; Rabelo, T.K.; da Rocha, R.F.; Quintans, L.; de Souza Araújo, A.A.; da Silva, F.A.; et al. Redox Properties and Cytoprotective Actions of Atranorin, a Lichen Secondary Metabolite. Toxicol. Vitr. 2011, 25, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Lohezic-Le Devehat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic Extracts and Metabolites as UV Filters. J. Photochem. Photobiol. B 2013, 120, 17–28. [Google Scholar] [CrossRef]
- De Melo, M.G.D.; Araújo, A.A.d.S.; Serafini, M.R.; Carvalho, L.F.; Bezerra, M.S.; Ramos, C.S.; Bonjardim, L.R.; Albuquerque-Júnior, R.L.C.; Lima, J.T.; Siqueira, R.S.; et al. Anti-Inflammatory and Toxicity Studies of Atranorin Extracted from Cladina kalbii Ahti in Rodents. Braz. J. Pharm. Sci. 2011, 47, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, R.S.; Bonjardim, L.R.; Araújo, A.A.S.; Araújo, B.E.S.; Melo, M.G.D.; Oliveira, M.G.B.; Gelain, D.P.; Silva, F.A.; Desantana, J.M.; Albuquerque, R.L.C.; et al. Antinociceptive Activity of Atranorin in Mice Orofacial Nociception Tests. Z. Naturforsch. C J. Biosci. 2010, 65, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Pereira-Filho, R.N.; Quintans, J.S.S.; Barreto, A.S.; DeSantana, J.M.; Santana-Filho, V.J.; Santos, M.R.V.; Bonjardim, L.R.; Araújo, A.A.S.; et al. Evaluation of Wound Healing Activity of Atranorin, a Lichen Secondary Metabolite, on Rodents. Rev. Bras. Farmacogn. 2013, 23, 310–319. [Google Scholar] [CrossRef] [Green Version]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.M.; Suh, S.S.; Kim, T.K.; Kim, J.E.; Han, S.J.; Youn, U.J.; Yim, J.H.; Kim, I.C. Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules 2018, 23, 658. [Google Scholar] [CrossRef] [Green Version]
- Emsen, B.; Aslan, A.; Turkez, H.; Joughi, A.; Kaya, A. The Anti-Cancer Efficacies of Diffractaic, Lobaric, and Usnic Acid: In Vitro Inhibition of Glioma. J. Cancer Res. Ther. 2018, 14, 941–951. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant Activity of Some Lichen Metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Naaz, Q.; Iqbal Choudhary, M.; Ahmed Mesaik, M.; Karunaratne, V. Enzyme Inhibitory and Immunomodulatory Activities of the Depsidone Lobaric Acid Extracted from the Lichen Heterodermia Sp. J. Natl. Sci. Found 2014, 42, 193–196. [Google Scholar] [CrossRef]
- Carpentier, C.; Barbeau, X.; Azelmat, J.; Vaillancourt, K.; Grenier, D.; Lagüe, P.; Voyer, N. Lobaric Acid and Pseudodepsidones Inhibit NF-ΚB Signaling Pathway by Activation of PPAR-γ. Bioorg. Med. Chem. 2018, 26, 5845–5851. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, J.; Yim, J.H.; Lee, H.K.; Pyo, S. Anti-Inflammatory Activity of Lobaric Acid via Suppressing NF-ΚB/MAPK Pathways or NLRP3 Inflammasome Activation. Planta Med. 2019, 85, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Schinkovitz, A.; Le Pogam, P.; Derbré, S.; Roy-Vessieres, E.; Blanchard, P.; Thirumaran, S.L.; Breard, D.; Aumond, M.C.; Zehl, M.; Urban, E.; et al. Secondary Metabolites from Lichen as Potent Inhibitors of Advanced Glycation End Products and Vasodilative Agents. Fitoterapia 2018, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Putra, P.P.; Abdullah, S.S.; Rahmatunisa, R.; Junaidin, J.; Ismed, F. Structure, Activity, and Drug-Likeness of Pure Compounds of Sumatran Lichen (Stereocaulon halei) for the Targeted ACE2 Protein in COVID-19 Disease. Pharmaciana 2020, 10, 135. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological Activities and Chemical Composition of Lichens from Serbia. EXCLI J. 2014, 13, 1226–1238. [Google Scholar] [CrossRef]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative Stress Mediated by Gyrophoric Acid from the Lichen Umbilicaria Hirsuta Affected Apoptosis and Stress/Survival Pathways in HeLa Cells. BMC Complement. Altern. Med. 2019, 19, 221. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H. Anti-Aging Effects of Gyrophoric Acid on UVA-Irradiated Normal Human Dermal Fibroblasts. Nat. Prod. Commun. 2020, 15, 1934578X20919545. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective Activity and Cytotoxic Potential of Two Parmeliaceae Lichens: Identification of Active Compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In Vitro Neuroprotective Potential of Lichen Metabolite Fumarprotocetraric Acid via Intracellular Redox Modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Brandao, L.F.G.; Santos, N.P.d.S.; Pereira, E.C.G.; da Silva, N.H.; Matos, M.d.F.C.; Bogo, D.; Honda, N.K. Effects of Fumarprotocetraric Acid, a Depsidone from the Lichen Cladonia verticillaris, on Tyrosinase Activity. Orbital Electron. J. Chem. 2017, 9, 256–261. [Google Scholar] [CrossRef]
- De Barros Alves, G.M.; De Sousa Maia, M.B.; De Souza Franco, E.; Galvão, A.M.; Da Silva, T.G.; Gomes, R.M.; Martins, M.B.; Da Silva Falcão, E.P.; De Castro, C.M.M.B.; Da Silva, N.H. Expectorant and Antioxidant Activities of Purified Fumarprotocetraric Acid from Cladonia verticillaris Lichen in Mice. Pulm. Pharmacol. Ther. 2014, 27, 139–143. [Google Scholar] [CrossRef]
- González, C.; Cartagena, C.; Caballero, L.; Melo, F.; Areche, C.; Cornejo, A. The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells. Molecules 2021, 26, 3760. [Google Scholar] [CrossRef]
- Russo, A.; Caggia, S.; Piovano, M.; Garbarino, J.; Cardile, V. Effect of Vicanicin and Protolichesterinic Acid on Human Prostate Cancer Cells: Role of Hsp70 Protein. Chem. Biol. Interact. 2012, 195, 1–10. [Google Scholar] [CrossRef]
- Shrestha, G.; El-Naggar, A.M.; St Clair, L.L.; O’Neill, K.L. Anticancer Activities of Selected Species of North American Lichen Extracts. Phytother. Res. 2015, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bessadottir, M.; Einarsdottir, E.; Jonsdottir, G.; Omarsdottir, S.; Ogmundsdottir, H.M. 870 The Lichen Compound Protolichesterinic Acid Affects Lipid Metabolism and Induces ER Stress in Cancer Cells. EJC Suppl. 2010, 8, 219–220. [Google Scholar] [CrossRef]
- Bessadóttir, M.; Skúladóttir, E.; Gowan, S.; Eccles, S.; Ómarsdóttir, S.; Ögmundsdóttir, H.M. Effects of Anti-Proliferative Lichen Metabolite, Protolichesterinic Acid on Fatty Acid Synthase, Cell Signalling and Drug Response in Breast Cancer Cells. Phytomedicine 2014, 21, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Bessadóttir, M.; Eiríksson, F.F.; Becker, S.; Ögmundsdóttir, M.H.; Ómarsdóttir, S.; Thorsteinsdóttir, M.; Ögmundsdóttir, H.M. Anti-Proliferative and pro-Apoptotic Effects of Lichen-Derived Compound Protolichesterinic Acid Are Not Mediated by Its Lipoxygenase-Inhibitory Activity. Prostaglandins Leukot. Essent. Fat. Acids 2015, 98, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Bellio, P.; Bozzi, A.; Amicosante, G.; Nicoletti, M.; Piovano, M.; Celenza, G. Protolichesterinic Acid Enhances Doxorubicin-Induced Apoptosis in HeLa Cells in Vitro. Life Sci. 2016, 158, 89–97. [Google Scholar] [CrossRef]
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia Prunastri and Pseudoevernia Furfuraceae Lichens and Their Major Metabolites as Antioxidant, Antimicrobial and Anticancer Agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Stojanović, I.; Najman, S.; Jovanović, O.; Petrović, G.; Najdanović, J.; Vasiljević, P.; Šmelcerović, A. Effects of Depsidones from Hypogymnia physodes on HeLa Cell Viability and Growth. Folia Biol. 2014, 60, 89. [Google Scholar]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic Activity of Physodic Acid and Acetone Extract from Hypogymnia physodes against Breast Cancer Cell Lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef] [Green Version]
- Emsen, B.; Sadi, G.; Bostanci, A.; Aslan, A. In Vitro Evaluation of Cytotoxic, Oxidative, Genotoxic, and Apoptotic Activities of Physodic Acid from Pseudevernia furfuracea in HepG2 and THLE2 Cells. Plant Biosyst. 2020, 155, 1111–1120. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component-Physodic Acid through the Blood-Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-Derived Caperatic Acid and Physodic Acid Inhibit Wnt Signaling in Colorectal Cancer Cells. Mol. Cell Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsen, B.; Turkez, H.; Togar, B.; Aslan, A. Evaluation of Antioxidant and Cytotoxic Effects of Olivetoric and Physodic Acid in Cultured Human Amnion Fibroblasts. Hum. Exp. Toxicol. 2017, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of Depsides and Depsidones from Lichen as Potent Inhibitors of Microsomal Prostaglandin E2 Synthase-1 Using Pharmacophore Models. ChemMedChem 2012, 7, 2077. [Google Scholar] [CrossRef] [PubMed]
- Brandão, L.F.G.; Alcantara, G.B.; De Fátima Cepa Matos, M.; Bogo, D.; Dos Santos Freitas, D.; Oyama, N.M.; Honda, N.K. Cytotoxic Evaluation of Phenolic Compounds from Lichens against Melanoma Cells. Chem. Pharm. Bull. 2013, 61, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Karagoz, I.D.; Ozaslan, M.; Guler, I.; Uyar, C.; Yalim, T.; Kazanci, U.; Aslan, A.; Cakir, A. In Vivo Antitumoral Effect of Diffractaic Acid from Lichen Metabolites on Swiss Albino Mice with Ehrlich Ascites Carcinoma: An Experimental Study. Int. J. Pharmacol. 2014, 10, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Maulidiyah, M.; Darmawan, A.; Ahmad, E.; Musdalifah, A.; Wibowo, D.; Salim, L.O.A.; Arham, Z.; Mustapa, F.; Nurdin, I.F.A.; Nurdin, M. Antioxidant Activity-Guided Isolation of Usnic Acid and Diffractaic Acid Compounds from Lichen Genus Usnea Sp. J. Appl. Pharm. Sci. 2020, 11, 075–083. [Google Scholar] [CrossRef]
- Bayir, Y.; Odabasoglu, F.; Cakir, A.; Aslan, A.; Suleyman, H.; Halici, M.; Kazaz, C. The Inhibition of Gastric Mucosal Lesion, Oxidative Stress and Neutrophil-Infiltration in Rats by the Lichen Constituent Diffractaic Acid. Phytomedicine 2006, 13, 584–590. [Google Scholar] [CrossRef]
- Karagoz, I.D.; Ozaslan, M.; Kilic, I.H.; Guler, I.; Uyar, C.; Tuter, D.; Kazanci, U.; Aslan, A.; Cakir, A.; Gezici, S. Hepatoprotective Effect of Diffractaic Acid on Carbon Tetrachloride-Induced Liver Damage in Rats. Biotechnol. Biotechnol. Equip. 2015, 29, 1011–1016. [Google Scholar] [CrossRef]
- Kosanić, M.M.; Ranković, B.R.; Stanojković, T.P. Antioxidant, Antimicrobial and Anticancer Activities of Three Parmelia Species. J. Sci. Food Agric. 2012, 92, 1909–1916. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid.-Based Complement. Altern. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomović, J.; Kosanić, M.; Ristić, S.; Ranković, B.; Stanojković, T.; Manojlović, N. Chemical Composition and Bioactive Properties of the Lichen. Pleurosticta acetabulum. Trop. J. Pharm. Res. 2018, 16, 2977–2984. [Google Scholar] [CrossRef] [Green Version]
- Manojlović, N.; Ranković, B.; Kosanić, M.; Vasiljević, P.; Stanojković, T. Chemical Composition of Three Parmelia Lichens and Antioxidant, Antimicrobial and Cytotoxic Activities of Some Their Major Metabolites. Phytomedicine 2012, 19, 1166–1172. [Google Scholar] [CrossRef]
- Alexandrino, C.A.F.; Honda, N.K.; Matos, M.d.F.C.; Portugal, L.C.; de Souza, P.R.B.; Perdomo, R.T.; Guimarães, R.d.C.A.; Kadri, M.C.T.; Silva, M.C.B.L.; Bogo, D. Antitumor Effect of Depsidones from Lichens on Tumor Cell Lines and Experimental Murine Melanoma. Rev. Bras. Farmacogn. 2019, 29, 449–456. [Google Scholar] [CrossRef]
- Amo de Paz, G.; Raggio, J.; Gómez-Serranillos, M.P.; Palomino, O.M.; González-Burgos, E.; Carretero, M.E.; Crespo, A. HPLC Isolation of Antioxidant Constituents from Xanthoparmelia Spp. J. Pharm. Biomed. Anal. 2010, 53, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, G.; Tinabaye, A.; Ananthi, R. In Vitro Antioxidant Activities of Salazinic Acid and its Derivative HexaacetylSalazinic Acid. Int. J. Eng. Res. Technol. 2015, 4, 345–355. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Elsayed, H.E.; Mohyeldin, M.M.; Akl, M.R.; Bhattacharjee, J.; Egbert, S.; El Sayed, K.A. Norstictic Acid Inhibits Breast Cancer Cell Proliferation, Migration, Invasion, and In Vivo Invasive Growth Through Targeting C-Met. Phytother. Res. 2016, 30, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Le Dévéhat, F.L.; Boustie, J.; Chakravarty, S. Lichen-Derived Compounds Show Potential for Central Nervous System Therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef]
- Taş, İ.; Han, J.; Park, S.Y.; Yang, Y.; Zhou, R.; Gamage, C.D.B.; Van Nguyen, T.; Lee, J.Y.; Choi, Y.J.; Yu, Y.H.; et al. Physciosporin Suppresses the Proliferation, Motility and Tumourigenesis of Colorectal Cancer Cells. Phytomedicine 2019, 56, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Taş, İ.; Varlı, M.; Son, Y.; Han, J.; Kwak, D.; Yang, Y.; Zhou, R.; Gamage, C.D.B.; Pulat, S.; Park, S.Y.; et al. Physciosporin Suppresses Mitochondrial Respiration, Aerobic Glycolysis, and Tumorigenesis in Breast Cancer. Phytomedicine 2021, 91, 153674. [Google Scholar] [CrossRef]
- Palacios-Moreno, J.; Rubio, C.; Quilhot, W.; Cavieres, M.F.; de la Peña, E.; Quiñones, N.V.; Díaz, H.; Carrión, F.; Henríquez-Roldán, C.F.; Weinstein-Oppenheimer, C.R.; et al. Epanorin, a Lichen Secondary Metabolite, Inhibits Proliferation of MCF-7 Breast Cancer Cells. Biol. Res. 2019, 52, 55. [Google Scholar] [CrossRef] [Green Version]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological Activities of Toninia candida and Usnea barbata Together with Their Norstictic Acid and Usnic Acid Constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [Green Version]
- Emsen, B.; Aslan, A.; Togar, B.; Turkez, H. In Vitro Antitumor Activities of the Lichen Compounds Olivetoric, Physodic and Psoromic Acid in Rat Neuron and Glioblastoma Cells. Pharm. Biol. 2016, 54, 1748–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Piovano, M.; Lombardo, L.; Garbarino, J.; Cardile, V. Lichen Metabolites Prevent UV Light and Nitric Oxide-Mediated Plasmid DNA Damage and Induce Apoptosis in Human Melanoma Cells. Life Sci. 2008, 83, 468–474. [Google Scholar] [CrossRef]
- Varol, M.; Türk, A.; Candan, M.; Tay, T.; Koparal, A.T. Photoprotective Activity of Vulpinic and Gyrophoric Acids Toward Ultraviolet B-Induced Damage in Human Keratinocytes. Phytother. Res. 2016, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Dabagoglu Psav, S.; Avan, I.; Candan, M.; Sahinturk, V.; Koparal, A.T. Vulpinic Acid, a Lichen Metabolite, Emerges as a Potential Drug Candidate in the Therapy of Oxidative Stress-Related Diseases, Such as Atherosclerosis. Hum. Exp. Toxicol. 2019, 38, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, N.; Aras, S.; Cansaran-Duman, D. Determination of Vulpinic Acid Effect on Apoptosis and MRNA Expression Levels in Breast Cancer Cell Lines. Anticancer Agents Med. Chem. 2018, 18, 2032–2041. [Google Scholar] [CrossRef]
- Kiliç, N.; Derici, M.K.; Büyük, I.; Aydin, S.S.; Aras, S.; Cansaran-Duman, D. Evaluation of in Vitro Anticancer Activity of Vulpinic Acid and Its Apoptotic Potential Using Gene Expression and Protein Analysis. Indian J. Pharm. Educ. Res. 2018, 52, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Cansaran-Duman, D.; Guney Eskiler, G.; Colak, B.; Sozen Kucukkara, E. Vulpinic Acid as a Natural Compound Inhibits the Proliferation of Metastatic Prostate Cancer Cells by Inducing Apoptosis. Mol. Biol. Rep. 2021, 48, 6025–6034. [Google Scholar] [CrossRef]
- Dailey, R.N.; Montgomery, D.L.; Ingram, J.T.; Siemion, R.; Vasquez, M.; Raisbeck, M.F. Toxicity of the Lichen Secondary Metabolite (+)-Usnic Acid in Domestic Sheep. Vet. Pathol. 2008, 45, 19–25. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.A.; Frankos, V.H. Review of Usnic Acid and Usnea barbata Toxicity. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Sonko, B.J.; Schmitt, T.C.; Guo, L.; Shi, Q.; Boros, L.G.; Leakey, J.E.A.; Beger, R.D. Assessment of Usnic Acid Toxicity in Rat Primary Hepatocytes Using 13C Isotopomer Distribution Analysis of Lactate, Glutamate and Glucose. Food Chem. Toxicol. 2011, 49, 2968–2974. [Google Scholar] [CrossRef] [Green Version]
- Frankos, V.H. NTP Nomination for Usnic Acid and Usnea barbata; National Toxicology Program, U.S. Department of Health and Human Services: Washington, DC, USA, 2004.
- Favreau, J.T.; Ryu, M.L.; Braunstein, G.; Orshansky, G.; Park, S.S.; Coody, G.L.; Love, L.A.; Fong, T.L. Severe Hepatotoxicity Associated with the Dietary Supplement LipoKinetix. Ann. Intern. Med. 2002, 136, 590–595. [Google Scholar] [CrossRef] [PubMed]
- García-Cortés, M.; Robles-Díaz, M.; Ortega-Alonso, A.; Medina-Caliz, I.; Andrade, R.J. Hepatotoxicity by Dietary Supplements: A Tabular Listing and Clinical Characteristics. Int. J. Mol. Sci. 2016, 17, 537. [Google Scholar] [CrossRef] [Green Version]
- Pramyothin, P.; Janthasoot, W.; Pongnimitprasert, N.; Phrukudom, S.; Ruangrungsi, N. Hepatotoxic Effect of (+)Usnic Acid from Usnea Siamensis Wainio in Rats, Isolated Rat Hepatocytes and Isolated Rat Liver Mitochondria. J. Ethnopharmacol. 2004, 90, 381–387. [Google Scholar] [CrossRef]
- Moreira, C.T.; Oliveira, A.L.; Comar, J.F.; Peralta, R.M.; Bracht, A. Harmful Effects of Usnic Acid on Hepatic Metabolism. Chem. Biol. Interact. 2013, 203, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Matsumaru, K.; Rettori, D.; Kaplowitz, N. Usnic Acid-Induced Necrosis of Cultured Mouse Hepatocytes: Inhibition of Mitochondrial Function and Oxidative Stress. Biochem. Pharmacol. 2004, 67, 439–451. [Google Scholar] [CrossRef]
- Venkataramana, D.; Krishna, D.R. Pharmacokinetics of d(+)-Usnic Acid in Rabbits after Intravenous Administration. Eur. J. Drug Metab. Pharmacokinet. 2010, 18, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.A.; Salakhutdinov, N.F. Biological Activity of Usnic Acid and Its Derivatives: Part 2. Effects on Higher Organisms. Molecular and Physicochemical Aspects. Russ. J. Bioorganic. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Krishna, D.R.; Ramana, D.V.; Mamidi, N.V.S.R. In Vitro Protein Binding and Tissue Distribution of D(+) Usnic Acid. Drug Metabol. Drug Interact. 1995, 12, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Dickmann, L.J.; Davis, J.A.; Greene, R.J.; Hill, J.J.; Howard, M.L.; Pearson, J.T.; Rock, D.A.; Tay, J.C.; Wahlstrom, J.L.; et al. Metabolism and Related Human Risk Factors for Hepatic Damage by Usnic Acid Containing Nutritional Supplements. Xenobiotica 2008, 38, 264–280. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R. Role of the NF-KappaB Pathway in the Pathogenesis of Human Disease States. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Li, Y.L.; Zhao, F.C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Miralles, I.; Edwards, H.G.M.; Domingo, F.; Jorge-Villar, S.E. Lichens around the World: A Comprehensive Study of Lichen Survival Biostrategies Detected by Raman Spectroscopy. Anal. Methods 2015, 7, 6856–6868. [Google Scholar] [CrossRef]
- Davis, R.L. Mechanism of Action and Target Identification: A Matter of Timing in Drug Discovery. iScience 2020, 23, 101487. [Google Scholar] [CrossRef]
- Uetrecht, J.; Naisbitt, D.J. Idiosyncratic Adverse Drug Reactions: Current Concepts. Pharmacol. Rev. 2013, 65, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic Acid and Its Derivatives for Pharmaceutical Use: A Patent Review (2000–2017). Expert. Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- da Silva Santos, N.P.; Nascimento, S.C.; Wanderley, M.S.O.; Pontes-Filho, N.T.; da Silva, J.F.; de Castro, C.M.M.B.; Pereira, E.C.; da Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. Nanoencapsulation of Usnic Acid: An Attempt to Improve Antitumour Activity and Reduce Hepatotoxicity. Eur. J. Pharm. Biopharm. 2006, 64, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.C.S.; Almeida, F.J.F.; Wanderley, M.S.O.; Ferraz, M.S.; Santos, N.P.S.; López, A.M.Q.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Usnic Acid: From an Ancient Lichen Derivative to Promising Biological and Nanotechnology Applications. Phytochem. Rev. 2020, 20, 609–630. [Google Scholar] [CrossRef]



| Secondary Metabolite | Lichen Species |
|---|---|
| Usnic acid | Cladonia lepidophora [25], Cetraria aculeata [25,29], Hypogymnia lugubris [27], Ochrolechia antarctica [25,29], Ochrolechia frigida [25], Parmelia saxatilis [25], Protousnea malacea [23], Protousnea magellanica [24], Psoroma hypnorum [25,29], Ramalina terebrata [25,29], Rhizoplaca aspidophora [25,29], Sphaerophorus globosus [25,29], Stereocaulon alpinum [25,29], Umbilicaria antarctica [25]. |
| Atranorin | Buellia cladocarpiza [25,29], Catillaria corymbose [25,29], Cladonia cornuta [25,31], Cladonia gracilis [25,31], Haematomma erythromma [25,31], Hypogymnia lugubris [27], Lecanora atra [25,29], Parmelia saxatilis [25,29], Psoroma contextum [30], Psoroma hypnorum [25,29,31], Psoroma tenue [25,29,30], Stereocaulon alpinum [25], Umbilicaria antarctica [25]. |
| Norstictic acid | Acarospora macrocyclos [25,31], Bryoria chalybeiformis [25,31], Psoroma genus (P. contextum, P. hypnorum, P. tenue) [25,30], Rinodina petermanii [25,31]. |
| Gyrophoric acid | Ochrolechia frígida [25], Ochrolechia deceptionis [25,29,31], Placopsis contortuplicata [25], Umbilicaria antarctica [25]. |
| Vicanicin | Erioderma chilense [28], Psoroma genus (P. contortum, P. dimorphum, P. leprolomun, P. microphyllizans, P. pallidum, P. pholidotoides, P. pulchrum, P. sphinctrinum, P. soccatum) [26,30]. |
| Protolichesterinic acid | Cetraria aculeata [25,29]. |
| Fumarprotocetraric acid | Cladonia cornuta [25,29]. |
| Diffractaic acid | Protousnea magellanica [25]. |
| Sphaerophorin | Sphaerophorus globosus [25,29]. |
| Psoromic acid | Rhizocarpon geographicum [25,31]. |
| Variolaric acid | Ochrolechia antarctica [25,29], Ochrolechia deceptionis [25,29,31]. |
| Lobaric acid | Stereocaulon alpinum [25,29]. |
| Salazinic acid | Parmelia saxatilis [25,29]. |
| Physodic acid | Hypogymnia lugubris [27]. |
| Cancer Cell Line | Evaluated Time (H) | IC50 Results (µg/mL) | References |
|---|---|---|---|
| LS174 (colon carcinoma) | 72 | 17.89 | [86] |
| FemX (human melanoma) | 72 | 19.52 | [86] |
| MCF-7 (breast cancer) | 72 | 34.06 | [88] |
| T47D (breast cancer) | 72 | 35.47 | [88] |
| MDA-MB-231 (breast cancer) | 72 | 44.18 | [88] |
| A-172 (glioblastoma multiforme) | 48 | 61.37 | [90] |
| HeLa (cervical cancer) | 72 | 66 | [87] |
| U-138MG (glioblastoma multiforme) | 48 | 68.36 | [90] |
| T98G (glioblastoma multiforme) | 48 | 72.15 | [90] |
| HepG2 (hepatic cancer) | 72 | 166.15 | [89] |
| Secondary Metabolite | Associated Species | Documented Biological Activities |
|---|---|---|
| Divaricatic acid | Protousnea malacea, Lecanora frustulosa |
|
| Evernic acid | Evernia prunastri |
|
| Lecanoric acid | Umbilicaria antarctica, Ochrolechia androgyna |
|
| Norstictic acid | Toninia candida, Xanthoparmelia chlorochroa, Parmotrema, Pseudoparmelia, and Usnea spp. | |
| Olivetoric acid | Pseudevernia furfuracea | |
| Pannarin | Sphaerophorus globosus, Psoroma genus | |
| Perlatolic acid | Cladonia portentosa | |
| Psoromic acid | Alectoria, Psoroma, and Usnea spp. | |
| Rhizocarpic acid | Rhizocarpon geographicum |
|
| Sekikaic acid | Protousnea malacea | |
| Sphaerophorin | Sphaerophorus globosus. | |
| Variolaric acid | Ochrolechia spp. | |
| Vicanicin | Psoroma spp. | |
| Vulpinic acid | Vulpicida pinastri |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulsen-Silva, E.; Gordillo-Fuenzalida, F.; Atala, C.; Moreno, A.A.; Otero, M.C. Bioactive Lichen Secondary Metabolites and Their Presence in Species from Chile. Metabolites 2023, 13, 805. https://doi.org/10.3390/metabo13070805
Poulsen-Silva E, Gordillo-Fuenzalida F, Atala C, Moreno AA, Otero MC. Bioactive Lichen Secondary Metabolites and Their Presence in Species from Chile. Metabolites. 2023; 13(7):805. https://doi.org/10.3390/metabo13070805
Chicago/Turabian StylePoulsen-Silva, Erick, Felipe Gordillo-Fuenzalida, Cristian Atala, Adrián A. Moreno, and María Carolina Otero. 2023. "Bioactive Lichen Secondary Metabolites and Their Presence in Species from Chile" Metabolites 13, no. 7: 805. https://doi.org/10.3390/metabo13070805
APA StylePoulsen-Silva, E., Gordillo-Fuenzalida, F., Atala, C., Moreno, A. A., & Otero, M. C. (2023). Bioactive Lichen Secondary Metabolites and Their Presence in Species from Chile. Metabolites, 13(7), 805. https://doi.org/10.3390/metabo13070805