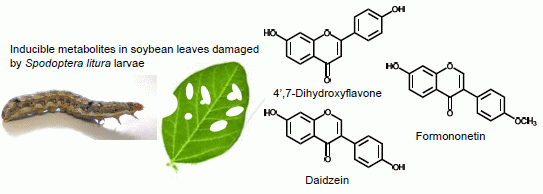
Insect-Induced Daidzein, Formononetin and Their Conjugates in Soybean Leaves
Abstract
:1. Introduction
2. Results
2.1. Metabolites Induced by S. litura Herbivory





2.2. Metabolites Induced by S. litura Gut Contents

2.3. Gut Content Extracts Treatment with Labeled Phenylalanine

3. Discussion
4. Materials and Methods
4.1. Plants and Insects
4.2. Chemicals
4.3. Feeding Treatment
4.4. Gut Contents Treatment
4.5. Phenylalanine Derivatization
4.6. Extraction and Sample Preparations
4.7. Instruments for Chemical Analysis
4.8. Preparation of Ononin and Malonylononin
4.9. Statistical Analysis
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ingham, J.L.; Keen, N.T.; Mulheirn, L.J.; Lyne, R.L. Inducibly-formed isoflavonoids from leaves of soybean. Phytochemistry 1981, 20, 795–798. [Google Scholar] [CrossRef]
- Osman, S.F.; Fett, W.F. Isoflavone glucoside stress metabolites of soybean Leaves. Phytochemistry 1983, 22, 1921–1923. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Yang, X.B.; Martinson, C.A.; Murphy, P.A. Effects of wounding and inoculation with Sclerotina sclerotiorum on isoflavone concentrations in soybean. Can. J. Plant Sci. 2005, 749–760. [Google Scholar]
- Graham, T.L.; Kim, J.E.; Graham, M.Y. Role of constitutive isoflavone conjugates in the accumulation of glyceollin in soybean infected with Phytophthora megasperma. Mol. PlantMicrobe Interact. 1990, 3, 157–166. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Cahill, D.M.; Bhattacharyya, M.K. Abscisic acid supplession of phenylalanine ammonia-lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1989, 91, 23–27. [Google Scholar] [CrossRef]
- Barz, W.; Well, R. Biosynthesis and metabolism of isoflavones and pterocarpan phytoalexins in chickepea, soybean and phytopathogenic fungi. Recent Adv. Phytochem. 1992, 26, 139–164. [Google Scholar]
- Graham, T.L. Flavonoid and isoflavonoid distribution in developing soybean seeding tissues and in seed and root exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]
- Graham, T.L.; Graham, M.Y. Glyceollin elicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations. Mol. Plantmicrobe Interact. 1991, 4, 60–68. [Google Scholar] [CrossRef]
- Morris, P.F.; Savard, M.E.; Ward, E.W.B. Identification and accumulation of isoflavonoids and isoflavone glucosides in soybean leaves hypocotyls in resistance responses to Phytophthora megasperma f. sp. glycinea. Physiol. Mol. Plant Pathol. 1991, 39, 229–244. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Ann. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Mithofer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Ann. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Glauser, G.; Marti, G.; Villard, N.; Doyen, G.A.; Wolfender, J.L.; Turlings, T.C.J.; Erb, M. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011, 68, 901–911. [Google Scholar] [CrossRef]
- Macias, F.A.; Martin, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G. Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat. Prod. Rep. 2009, 26, 478–489. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3( 4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Iwamura, H. Induction of HDMBOA-Glc accumulation and DIMBOA Glc 4-O-methyltransferase by jasmonic acid in poaceous plants. Phytochemistry 2002, 61, 331–337. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Tanaka, C.; Mori, N.; Tsuda, M.; Iwamura, H. Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves. Phytochemistry 2004, 65, 2995–3001. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate-myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef]
- Piubelli, G.C.; Hoffmann-Campo, C.B.; De Arruda, I.C.; Franchini, J.C.; Lara, F.M. Flavonoid increase in soybean as a response to Nezara viridura injury and its effect on insect-feeding preference. J. Chem. Ecol. 2003, 29, 1223–1233. [Google Scholar]
- Alborn, H.T.; Turlings, T.C.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–948. [Google Scholar] [CrossRef]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E.A. Disulfooxy fatty acids from the american bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. β-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef]
- Roda, A.; Halitschke, R.; Steppunhn, A.; Baldwin, I.T. Individual variability in herbivore-specific elicitors from the plant’s perspective. Mol. Ecol. 2004, 13, 2421–2433. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E.A. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Graham, T.L. Partial purification and characterization of a soybean β-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry 2001, 58, 995–1005. [Google Scholar] [CrossRef]
- Alborn, H.T.; Brennan, M.M.; Tumlinson, J.H. Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae. J. Chem. Ecol. 2003, 29, 1357–1372. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C. Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc. Natl. Acad. Sci. USA 2004, 101, 8993–8997. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef]
- Mori, N.; Alborn, H.T.; Teal, P.E.A.; Tumlinson, J.H. Enzymatic decomposition of elicitors of plant volatiles in Heliothis virescens and Helicoverpa zea. J. Insect Physiol. 2001, 47, 749–757. [Google Scholar] [CrossRef]
- Mori, N.; Yoshinaga, N.; Sawada, Y.; Fukui, M.; Shimoda, M.; Fujisaki, K.; Nishida, R.; Kuwahara, Y. Identification of volicitin-related compounds from the regurgitant of lepidopteran caterpillars. Biosci. Biotech. Biochem. 2003, 67, 1168–1171. [Google Scholar] [CrossRef]
- Mori, N.; Yoshinaga, N. Function and evolutionary diversity of fatty acid amino acid conjugates in insects. J. Plant Interact. 2011, 6, 103–107. [Google Scholar] [CrossRef]
- Pohnert, G.; Jung, V.; Haukioja, E.; Lempa, K.; Bolnad, W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 1999, 55, 11275–11280. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Alborn, H.T.; Nakanishi, T.; Suckling, D.M.; Nishida, R.; Tumlinson, J.H.; Mori, N. Fatty acid-amino acid conjugates diversification in lepidopteran caterpillars. J. Chem. Ecol. 2010, 36, 319–325. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H.; Teal, P.E.A. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar]
- Röse, U.S.R.; Manukian, A.; Heath, R.R.; Tumlinson, J.H. Volatile semiochemicals released from undamaged cotton leaves. Plant Physiol. 1996, 111, 487–495. [Google Scholar]
- Wink, M.; Roberts, M.F. Compartmentation of alkaloid synthesis, transport, and storage. In Alkaloids; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 239–262. [Google Scholar]
- Banks, S.; Dewick, P.M. Biosynthesis of glyceollins I, II and III in soybean. Phytochemistry 1983, 22, 2729–2733. [Google Scholar] [CrossRef]
- Ebel, J. Phytoalexin synthesis: The biochemical analysis of the induction process. Ann. Rev. Phytopathol. 1986, 24, 235–264. [Google Scholar] [CrossRef]
- Keen, N.T.; Sims, J.; Erwin, D.C.; Rice, E.; Partridge, J.E. 6a-Hydroxyphaseolllin: An antifungal chemical induced in soybean hypocotyls by Phytophthora megasperma var. sojae. Phytopathology 1971, 61, 1084–1089. [Google Scholar] [CrossRef]
- Welle, R.; Grisebach, H. Induction of phytoalexin synthesis ins oybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers. Arch. Biochem. Biophysiol. 1988, 263, 191–198. [Google Scholar] [CrossRef]
- Misaki, A.; Sekiya, K.; Yamatoya, K. Agent for inducing phytoalexin and method for inducing phytoalexin. U.S. Patent 5602111, 11 February 1997. [Google Scholar]
- West, C.A. Fungal elicitors of the phytoalexin response in higher plants. Naturwissenschaften 1981, 68, 447–457. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Luo, S.H.; Yi, T.S.; Luo, Q.; Hua, J.; Liu, Y.; Li, S.H. Secondary metabolites from Glycine soja and their growth inhibitory effects against Spodoptera litura. J. Agric. Food Chem. 2011, 59, 6004–6010. [Google Scholar] [CrossRef]
- Boughton, B.A.; Callahan, C.H.; Silva, C.; Bowne, J.; Nahid, A.; Rupasinghe, T.; Tull, D.L.; McConville, M.J.; Bacic, A.; Roessner, U. Comprehensive profiling and quantitation of amine group containing metabolites. Anal. Chem. 2011, 83, 7523–7530. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murakami, S.; Nakata, R.; Aboshi, T.; Yoshinaga, N.; Teraishi, M.; Okumoto, Y.; Ishihara, A.; Morisaka, H.; Huffaker, A.; Schmelz, E.A.; et al. Insect-Induced Daidzein, Formononetin and Their Conjugates in Soybean Leaves. Metabolites 2014, 4, 532-546. https://doi.org/10.3390/metabo4030532
Murakami S, Nakata R, Aboshi T, Yoshinaga N, Teraishi M, Okumoto Y, Ishihara A, Morisaka H, Huffaker A, Schmelz EA, et al. Insect-Induced Daidzein, Formononetin and Their Conjugates in Soybean Leaves. Metabolites. 2014; 4(3):532-546. https://doi.org/10.3390/metabo4030532
Chicago/Turabian StyleMurakami, Shinichiro, Ryu Nakata, Takako Aboshi, Naoko Yoshinaga, Masayoshi Teraishi, Yutaka Okumoto, Atsushi Ishihara, Hironobu Morisaka, Alisa Huffaker, Eric A Schmelz, and et al. 2014. "Insect-Induced Daidzein, Formononetin and Their Conjugates in Soybean Leaves" Metabolites 4, no. 3: 532-546. https://doi.org/10.3390/metabo4030532
APA StyleMurakami, S., Nakata, R., Aboshi, T., Yoshinaga, N., Teraishi, M., Okumoto, Y., Ishihara, A., Morisaka, H., Huffaker, A., Schmelz, E. A., & Mori, N. (2014). Insect-Induced Daidzein, Formononetin and Their Conjugates in Soybean Leaves. Metabolites, 4(3), 532-546. https://doi.org/10.3390/metabo4030532