Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview
Abstract
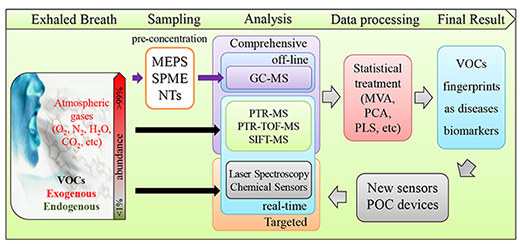
:1. Introduction
2. Exhaled Breath (EB) Analysis
2.1. EB Analysis Experimental Layout

2.1.1. EB Sampling

2.1.2. Pre-Concentration
2.2. EB Analysis
2.2.1. Off-line Analysis: Gas-Chromatography (GC)
| Target VOCs (Putative Biomarkers) (LODs) | Methodology | Sample (Patients/Controls) | Sensitivity/Specificity (%) | Statistical Approach (Pre-Processing Method; Classification Method; Performance Measures) | Reference |
|---|---|---|---|---|---|
| Oncologic Diseases | |||||
| Lung cancer (LC) | |||||
| 1-octene | SPME/GC-MS Chemical nanoarrays | 72/10 | DFA model: 86.0/96.0 Cross-validation: 86.0/88.0 | LDA; Wilcoxon/ Kruskal-Wallis ANOVA | [82] |
| isoprene (81.5 ppb), acetone (458.7 ppb), methanol (118.5 ppb) | PTR-MS/GC-MS | 285/472 | 4 compounds: 52.0/100; 15 (or 21) compounds: 71.0 (80.0)/100 | Kruskal-Wallis ANOVA | [30] |
| isoprene (6041 pM), pentane (647.5 pM), heptane (13.5 pM), octane (61.0 pM), styrene (17.9 pM), among 13 VOCs | SPME/GC-MS | 36/50 | 72.2/93.6 | Kolmogorov-Smirnov; ANOVA, Games Howell post-hoctest; Kruskal-Wallis ANOVA, Dunn’s Post Hoc test; Student t-test; p-value; PRISM | [83] |
| 2-butanone (1.78–8.38 nM), 2-hydroxyacetaldehyde (0.13–0.77 nM), 3-hydroxy-2-butanone (0.23–1.13 nM), 4-hydroxyhexenal (0.005–0.05 nM) | FT-ICR-MS | 97/88 | 89.8/81.3 | Wilcoxon (Minitab) | [39] |
| formaldehyde (7 ppb) | PTR-MS | 17/170 | 54.0/99.0 | FQDM; p-values from Wilcoxon; ROC; MATLAB (classify.m) | [84] |
| pentanal (0.001 nM), hexanal (0.010 nM), octanal (0.009 nM), nonanal (0.028 nM) | OFD-SPME/GC-MS | 12/12,12 | C5: 75.0/95.5; C6: 8.3/91.7 C8: 58.3/91.7; C9: 33.3/95.8 | Kruskal-Wallis ANOVA | [85] |
| ethane | GC-FID | 26/14 | - | ANOVA with Bonferroni’s correction for multiple comparisons | [86] |
| isoprene (0.095 nM), acetone (0.985 nM), 2-butanone (0.158 nM), ethanol (5.098 nM), acetaldehyde (1.280 nM), pentanal (0.436 nM), dimethyl sulphide (0.270 nM), pentane (0.431 nM) | SPME/GC-MS | 31/31,31 | - | PCA, Mann-Whitney Rank; Kruskal-Wallis ANOVA; post hoc Student-Newman-Keuls; Dunn’s Method | [87] |
| hexane, methylpentane, o-toluidine, aniline, alcohols, ketones | e-nose, GC-MS | 42/18 | Good | PLS-DA | [88] |
| styrene, decane, isoprene, benzene, undecane, 1-hexene, hexanal, propyl benzene, 1,2,4-trimethyl benzene, heptanal, methyl cyclopentane | SPME, virtual SAW gas sensor | 20,7/15 | Good | ANN | [89] |
| isobutene, methanol, ethanol, acetone, pentane, isoprene, isopropanol , dimethylsulfide, carbon disulphide, benzene, toluene | e-nose, GC-MS | 14/45; 14/62 | 71.4/91.9 | PCA, CDA, SVM | [90] |
| VOCs pattern recognition | colorimetric sensors | 49,18,15, 20,20/21 | Model validation: 73.3/72.4 21 patients: 100/60.0 | Random forest classifier | [91] |
| VOCs pattern recognition | e-nose | 10,10/10 | - | PCA, LDA, MVA, CVV, Savitzky–Golay filtering | [92] |
| Set of 42 VOCs | gold nanoparticle sensors, SPME/GC-MS | 40/56 | - | PCA | [93] |
| VOCs pattern recognition | colorimetric sensors | 92/137 | High, several groups defined | Logistic prediction model | [94] |
| 2-hexanone, 3-heptanone; 2,2,4-Trimethyl-hexane | SPME/GC-MS, sensors | 12,4,–1 | 100/80 | LDA | [95] |
| VOCs profile | PTR-MS, SPME-GC-MS | 220/441, 65/31 | variable | - | [30] |
| Mesothelioma | |||||
| VOCs pattern recognition | e-nose | 38/42 | 95/88 | PCA, LDA; Inbuilt Savitzky–Golay filtering | [96] |
| cyclopentane (0.40 ng/L), cyclohexane (4.67 ng/L) | TD-GC-MS | 13 + 13/13 | 92.3/82.7 | PCA, DFA and CP-ANN; ANOVA | [97] |
| VOCs pattern recognition | e-nose | 13,13/13 | 92.3/82.7 | PCA, DA; MVA | [98] |
| Breast Cancer (BC) | |||||
| nonane; 5-methyl-tridecane; 3-methyl-undecane; 6-methyl-pentadecane; 2-methyl-propane; 3-methyl-nonadecane; 4-methyl-dodecane; 2-methyl-octane | TD-GC-MS | 51/102 | 94.1/73.8 | positive predictive value and negative predictive value | [99] |
| undecane, dodecane, tridecane, tetradecane, pentadecane, d-limonene | TD-GC-MS | 54/204 | 78.5/88.3 | ROC, MCCV, MVA algorithm employing WDA | [100] |
| 3,3-Dimethyl-pentane, 5-(2-Methylpropyl)-nonane, 2,3,4-Trimethyl-decane, 2-Amino-5-isopropyl-8-methyl-1-azulenecarbonitrile, 1-Iodo-nonane | GC-MS | 22/22 | - | PCA and cluster analysis | [101] |
| hexanal (3.75 ppbV), heptanal (3.22 ppbV), octanal (3.39 ppbV), nonanal (2.49 ppbV) | GC-MS | 22,17/24 | 72.7/91.7 | Fisher DA; leave-one-out (LOO) DA; Kruskal-Wallis ANOVA, ROC, AUC | [102] |
| VOCs profile (A: BC on biopsy/normal screening mammograms (scr mam), B: normal/abnormal scr mam, C: BC/no BC on biopsy) | POC device (TD-GC-SAW) | 37 + 35/172 | A (81.8/70), B (86.5, 66.7), C (75.8, 74.0) | C-statistic ([AUC] of [ROC]), MCCV, MVA algorithm cross validated with a LOO method | [103] |
| VOCs pattern recognition | e-nose | 16,13/7 | 94/80 | PCA, SVM, cross validation | [104] |
| Colorectal cancer (CRC) | |||||
| decanal; 1,3-dimethylbenzene; 1,2-pentadiene Cyclohexane; Methyl cyclohexane; 4-methyloctane | GC-MS | 37/41 | 86/83 | PNN validated by the LOO method | [27] |
| 10 discriminant VOCs | SPME/GC-MS | 20/20 | - | PCA, PLS-DA | [45] |
| 4 discriminant VOCs | GC-MS | 26/22 | - | PCA and cluster analysis | [101] |
| Gastric cancer | |||||
| 6 discriminant VOCs | sensors, GC-MS | 37,32, –61 | 89/90 | LDA; Wilcoxon/Kruskal-Wallis ANOVA | [105] |
| Head-and-neck cancer | |||||
| 8 discriminant VOCs | e-nose, GC–MS | 22,25/40 | 100/100 | PCA with ANOVA and Student | [106] |
| VOCs pattern recognition | e-nose | 36/23 | 90/80 | Logistic regression, ROC | [107] |
| Liver cancer | |||||
| 2,3-dihydro-benzofuran, methane-sulfonyl chloride; acetic acid; ethanol | sensor, GC-MS | 95.8/100 | LDA; Shapiro-Wilk, Wilcoxon/Kruskal-Wallis ANOVA | [108] | |
| hexanal; 1-octen-3-ol; octane | SPME/GC-MS | 18/19 | 100/100 | RSD; χ2 | [109] |
| 3-Hydroxy-2-butanone, styrene, and decane (set A: HCC patients/normal controls; B: cross-validation) | GC-MS | 30/27 + 36 | A: 86.7/91.7 B. 83.3/91.7 | ROC and DA using the defined markers | [110] |
| Pulmonary Diseases | |||||
| Airways inflammation | |||||
| VOCs pattern recognition | e-nose | 110/108 | 72.2/75.1 | k-NN voting rule to classify features extracted by PCA | [111] |
| Asthma | |||||
| Several discriminant VOCs, including acetone and many alkanes | e-nose, GC-MS | 20/20 | - | PCA; cross-validation value, LDA on principal component reduction, M-distance | [112] |
| decane; dodecane; tetradecane; 2-methyl-1,3-butadiene; 2,2-dimethylhexane; 2,4-dimethyloctane, 2,3,6-trimethyldecane | GC-MS | 35/15 | - | PLS-DA; Single factor ANOVA | [113] |
| nonane; 2,2,4,6,6-pentamethylheptane; decane; 3,6-dimethyldecane; dodecane; tetradecane | GC-MS | 32/27 | 96/95 | PLS-DA, Monte Carlo cross-validation (MCCV) statistics | [114] |
| Several discriminant VOCs | GC-MS | 63/57 | 89/95 | Stepwise DA; 20-fold CVV DA | [115] |
| VOCs pattern recognition | e-nose/GC-MS | 27/24 | High, several groups defined | PCA, ANN | [116] |
| 17 discriminant VOCs | GC-TOF-MS | 252 | high | Random Forests (RF) and dissimilarity PLS-DA | [117] |
| Acute Respiratory Distress Syndrome (ARDS) | |||||
| octane, acetaldehyde and 3-methylheptane | GC-MS | 23/53 | 90 | Kruskal-Wallis ANOVA (continuous variables), χ2 (categorical variables) | [118] |
| acetone, isoprene, n-Pentane | GC-FID/GC-MS | 19/18 | - | Mann-Whitney U-Wilcoxon rank sum test (unpaired samples), Wilcoxon matched-pairs signed-ranks test (paired samples) | [119] |
| Pulmonary embolism | |||||
| VOCs pattern recognition | e-nose | 40/20 | 85/65 | LDA, PCA, ROC | [120] |
| Pulmonary Tuberculosis | |||||
| 6 discriminant VOCs | GC/MS | 42/59 | 95.7/78.9 | Fuzzy logic, Pattern recognition analysis; PLS, HCA, PCA, k-NN; PC regression, ROC, SIMCA | [121] |
| VOCs pattern recognition | POC device (TD-GC-SAW) | 130/121 | 71.2/72 | MCCV, multivariate predictive algorithm; ROC | [122] |
| Alkanes and derivatives, cyclohexane and benzene derivatives | GC/MS | 226 | variable | MCCV | [123] |
| Chronic Obstructive Pulmonary Disease (COPD)/Emphysema | |||||
| Ethane (No steroid treatment—2.77 ± 0.25 ppb; Steroid-treated—0.48 ± 0.05 ppb) | GC-FID | 22/14 | - | p-value; ANOVA—two-way variance analysis | [124] |
| MDA (57.2 nM), hexanal (63.5 nM) heptanal (26.6 nM) | LC-MS/MS | 20/12,20 | - | p-value; Wilcoxon, Bland-Altman | [125] |
| Mass-spectra | PTR-MS | - | 43/161 | bootstrapped stepwise forward logistic regression | [126] |
| VOCs pattern recognition | eNose | 33/10 | 100/100 | LDA; Wilcoxon, k-fold cross-validation | [127] |
| VOCs profile | MCC/IMS | High, variable with statist. used | 30 + 54/35 | decision tree, naive Bayes, linear support vector machine (SVM), ANN, RF and radial SVM | [128] |
| Cystic Fibrosis (CF) | |||||
| pentane (0.36 ppb), dimethyl sulphide (3.9 ppb) | GC-MS | 20/20 | - | Wilcoxon; linear regression | [129] |
| carbonyl sulphide (110 ± 60 pptv), dimethyl sulphide (4.780 ± 1.350 pptv), carbon disulphide (26 ± 38 pptv) | GC-MS | 20/23 | - | Student; F-score method; Pearson; Fisher’s z-score | [130] |
| ethane (no steroid treatment—1.99 ± 0.20 ppb; steroid treatment—0.67 ± 0.11 ppb) | GC-FID | 23/14 | - | ANOVA with Bonferroni’s correction | [131] |
| Other Diseases | |||||
| Cardiovascular Diseases (CVDs) | |||||
| Acute decompensated heart failure (ADHF) | |||||
| acetone (256–1974 ppb), pentane (20–74 ppb) | SIFT-MS | 25/16 | - | MVA | [28] |
| acetone (3.7 ppb) | GC-MS | 59,30/20 | 83/100 | Kruskal-Wallis ANOVA | [132] |
| Cholesterol | |||||
| Isoprene | GC/MS, SIFT-MS | - | - | [29] | |
| Atherosclerosis | |||||
| trimethyl amine | GC, SIFT-MS | - | - | [29] | |
| Carbohydrate malabsorption/maldigestion | |||||
| Ethanethiol, dimethylsulfide | PTR-MS | - | - | [133] | |
| Liver dysfunctions | |||||
| Liver Cirrhosis | |||||
| 2-butanone (3.2 ± 0.5 ppbv), methanol (528 ± 218 ppbv), heptadienol (2.5 ± 1.4 ppbv), monoterpenes (6.7 ± 5 ppbv) | PTR-TOF-MS | 12/14 | 83/86 | p-value; DA; Wilcoxon, Pearson | [5] |
| Non-Alcoholic Fatty Liver Disease (NAFLD) | |||||
| acetone (71.7 ppb), isoprene (14.7 ppb), trimethylamine (5 ppb), acetaldehyde (35.1 ppb), pentane (13.3 ppb) | SIFT-MS | 37/23 | - | - | [134] |
| Alcoholic hepatitis (AH) | |||||
| 2-propanol, acetaldehyde, acetone, ethanol, pentane, trimethylamine | SIFT-MS | 40,40/43 | 90/80 | p-value; Kruskal-Wallis ANOVA, Pearson χ2, Spearman correlation | [135] |
| Propionic acidaemia | |||||
| 3-heptanone | PTR-MS and GC-MS | - | - | - | [133] |
| Diabetes mellitus | |||||
| acetone | SPME/GC-MS, SIFT-MS, laser spectroscopy | - | - | - | [29] |
| acetone | e-nose, SIFT-MS | 8 | - | - | [136] |
| acetone (160–862 ppb) | SIFT-MS | - | 97.9/100 | p-value; Non-parametric tests | [137] |
| acetone; isopropanol; toluene; m-xylene; 2,3,4-trimethylhexane; 2,6,8-trimethyldecane; tridecane and undecane | SPME/GC-MS | 48/39 | - | PCA, OPLS-DA; MVA, Wilcoxon | [138] |
| VOCs pattern recognition | e-nose | 117/108 | 87.7/86.9 | k-NN voting rule to classify features extracted by PCA | [111] |
| Chronic renal failure | |||||
| NO (39 ppb) | Ozone chemioluminescence | 40/28 | - | p-value; DA; χ2 | [139] |
| TMA (0.33 ppb) | TD-GC-MS | 14/9 | - | Wilcoxon | [43] |
| Uraemia | IMS/GC-MS | 28 + 26/28 | ANOVA, two-sided two-sample Student’s t-tests | [140] | |
| VOCs pattern recognition | e-nose | 110/108 | 86.6/83.5 | k-NN voting rule to classify features extracted by PCA | [111] |
| Crohn’s disease | |||||
| Set A (healthy controls/CD remission)- 6 discriminatory VOCs; Set B (healthy controls/active CD); set C (active CD/remission)- 10 discriminatory VOCs | GC-TOF-MS | 725/110 | A and B (96/97); C (81/80) | RF to the most discriminatory VOCs for the 3 groups; PCA on proximity matrix obtained from the RF model. | [141] |
| Helicobacter pylori infection | |||||
| 13C O2/12CO2 | Cavity Ring-Down Spectroscopy (NIR) | - | 100/100 | - | [142] |
| Schizophrenia | |||||
| ethane and pentane | TD-GC-MS | 28/15 | - | - | [143] |
2.2.2. Real-Time Analysis
2.2.2.1. Proton Transfer Reaction Mass Spectrometry (PTR-MS)
2.2.2.2. Selected Ion Flow Tube Mass Spectrometry (SIFT-MS)
2.2.2.3. Ion Mobility Spectrometry (IMS)
2.2.3. Targeted Breath Analysis
Electronic Noses (e-noses)
3. The Metabolics of EB Volatiles
3.1. Hydrocarbons
3.1.1. Saturated Hydrocarbons
3.1.2. Unsaturated Hydrocarbons
Isoprene
3.2. Ketones
3.2.1. Acetone


3.2.2. 2-Butanone
3.3. Nitrogen Containing Compounds
3.3.1. Nitric Oxide (NO)
3.3.2. Dimethylamine (DMA) and Trimethylamine (TMA)
3.3.3. Acetonitrile (ACN)
3.4. Aldehydes
3.4.1. Formaldehyde
3.4.2. Hexanal and Heptanal
3.5. Potential Use of Breath Analysis in Different Diseases
3.5.1. Oncologic Diseases
3.5.2. Pulmonary Diseases
3.5.3. Other Diseases
4. Data Analysis and Discriminatory Models Used in Breath Biomarker Research
4.1. Data Pre-Processing and Normalization
4.2. Data Analysis
5. Conclusions

Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Abbreviations
| ACN | acetonitrile |
| ADH | Alcohol dehydrogenase |
| ADHF | Acute decompensated heart failure |
| AIDS | acquired immunodeficiency syndrome |
| AH | Alcoholic hepatitis |
| ALDH | aldehyde dehydrogenase |
| ANNs | artificial neural networks |
| TD | Thermal desorption |
| ATP | Adenosine triphosphate |
| AUC | area under the curves |
| BADD | breath analysis based disease diagnosis |
| CAR | Carboxen |
| CF | cystic fibrosis |
| CKD | chronic kidney disease |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRC | colorectal cancer |
| CT | computed tomography |
| CVDs | Cardiovascular diseases |
| DMPP | dimethylallyl pyrophosphate |
| DMA | Dimethyl amine |
| DNA | Deoxyribonucleic acid |
| DVB | Divinylbenzene |
| EB | exhaled breath |
| EBC | exhaled breath condensate |
| EDRF | endothelium-derived relaxing factor |
| EESI-MS | Extractive ElectroSpray Ionization Mass Spectrometry |
| FDH | formaldehyde dehydrogenase |
| FENO | NO fractional concentration in exhaled breath |
| FID | Flame ionization detector |
| HS-SPME | head-space solid phase micro extraction |
| FQDM | Fisher’s Quadratic Discriminant Method |
| GC | gas chromatography |
| GC-FID | gas chromatography combined with Flame ionization detector |
| GC-IMS | gas chromatography combined with ion mobility spectrometer |
| GC-MS | gas chromatography combined with mass spectrometry |
| GC-TOF-MS | gas chromatography combined with time-of-flight mass spectrometry |
| GSH | glutathione |
| HCA | hierarchical clustering analysis |
| HF | Heart Failure |
| HMG-CoA reductase | 3-hydroxy-3-methyl-glutaryl-CoA reductase |
| IBD | Inflammatory Bowel Disease |
| ICU | Intensive care unit |
| IL-4 | interleukin-4 |
| IMS | ion mobility spectrometry |
| iNOS | inducible NO synthase |
| k-NN | k-nearest neighbour |
| LC | Lung cancer |
| LDA | linear discriminate analysis |
| LDCT | low dose computed tomography |
| LOD | limit of detection |
| MCCV | Monte Carlo cross-validation |
| MDA | malondialdehyde |
| MEPS | microextraction by packed sorbent |
| MLR | Multiple Linear Regressions |
| MVA | multivariated analysis |
| MS | mass spectrometry |
| m/z | mass to-charge ratio |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NDDs | neurodegenerative diseases |
| NIR | near infrared spectroscopy |
| NLST | National Lung Screening Trial |
| N2 | nitrogen |
| NNK | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone |
| NO | nitric oxide |
| NSCLC | Non-Small Cell Lung Carcinoma |
| NTD | Needle Trap Device |
| O2 | oxygen |
| ODs | Oncologic diseases |
| ppm | parts per million |
| ppb | parts per billion |
| ppt | parts per trillion |
| PCA | Principal Component Analysis |
| PDMS | Polydimethylsiloxane |
| PFA | perfluoroalkoxy polymer |
| PLS | Partial least-square |
| PNN | Probabilistic neural network |
| POC | point of care |
| PTFE | polytetrafluoroethylene |
| PVDC | polyvinylidene chloride |
| PVF | polyvinyl fluoride |
| PTR | proton transfer reaction |
| PTR-MS | proton transfer reaction with mass spectrometry |
| PTR-TOF-MS | proton transfer reaction with time-of-flight mass spectrometry |
| ROC | Receiver operator characteristic |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SIFT-MS | Selected ion flow tube mass spectrometry |
| SIMCA | soft independent modelling of class analogy |
| SPME | solid phase micro extraction |
| SVM | support vector machine |
| S/N | signal-to-noise ratio |
| TD-tubes | thermal desorption tubes |
| TGF-β | transforming growth factor |
| TMA | trimethyl amine |
| TNF | tumour necrosis factor |
| TOF-MS | proton transfer reaction with time-of-flight mass spectrometry |
| VOCs | volatile organic compounds |
| WDA | weighted digital analysis |
Conflicts of Interest
References
- Boutayeb, A.; Boutayeb, S. The burden of non communicable diseases in developing countries. Int. J. Equity Health 2005. [Google Scholar] [CrossRef] [Green Version]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world--a growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Krisher, S.; Riley, A.; Mehta, K. Designing breathalyser technology for the developing world: How a single breath can fight the double disease burden. J. Med. Eng. Technol. 2014, 38, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Cavaco, C.; Perestrelo, R.; Pereira, J.; Câmara, J. Microextraction by packed sorbent (meps) and solid-phase microextraction (spme) as sample preparation procedures for the metabolomic profiling of urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; Marmo, R.; Caporaso, N.; Biasioli, F. Rapid "breath-print" of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS One 2013, 8, e59658. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology--gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Patel, K. Noninvasive tools to assess liver disease. Curr. Opin. Gastroenterol. 2010, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.S.; Ahmed, I.; Khalid, T.; Johnson, E.; Smith, S.; Ratcliffe, N. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointestin. Liver Dis. 2009, 18, 337–343. [Google Scholar] [PubMed]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Schubert, J.; Miekisch, W.; Geiger, K.; Nöldge-Schomburg, G. Breath analysis in critically ill patients: Potential and limitations. Expert Rev. Mol. Diagn. 2004, 4, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Risby, T.H.; Solga, S.F. Current status of clinical breath analysis. Appl. Phys. B 2006, 85, 421–426. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC, Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Tittel, F.K. Current status of midinfrared quantum and interband cascade lasers for clinical breath analysis. Opt. Eng. 2010, 49, 111123. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Minh Tdo, C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O'Neill, H.J. Volatile organic compounds in exhaled air from patients with lung cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar] [PubMed]
- O'Neill, H.J.; Gordon, S.M.; O'Neill, M.H.; Gibbons, R.D.; Szidon, J.P. A computerized classification technique for screening for the presence of breath biomarkers in lung cancer. Clin. Chem. 1988, 34, 1613–1618. [Google Scholar] [PubMed]
- Fenske, J.D.; Paulson, S.E. Human breath emissions of vocs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.A.; Liu, H.; Haick, H. Breath testing: The future for digestive cancer detection. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 389–391. [Google Scholar] [CrossRef] [PubMed]
- van de Kant, K.D.; van der Sande, L.J.; Jobsis, Q.; van Schayck, O.C.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: A systematic review. Respir. Res. 2012, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Miekisch, W.; Pleil, J.; Risby, T.; Schubert, J. Methodological issues of sample collection and analysis of exhaled breath. In Methodological issues for breath analysis; Maney Publishing: Leeds, UK, 2010; Volume 49, pp. 96–114. [Google Scholar]
- Amann, A.; Poupart, G.; Telser, S.; Ledochowski, M.; Schmid, A.; Mechtcheriakov, S. Applications of breath gas analysis in medicine. Int. J. Mass Spectrom. 2004, 239, 227–233. [Google Scholar] [CrossRef]
- Libardoni, M.; Stevens, P.T.; Waite, J.H.; Sacks, R. Analysis of human breath samples with a multi-bed sorption trap and comprehensive two-dimensional gas chromatography (gcxgc). J. Chromatogr. B 2006, 842, 13–21. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.A.; Tang, W.H.; Cikach, F., Jr.; Gul, Z.; Tranchito, L.; Paschke, K.M.; Viterna, J.; Wu, Y.; Laskowski, D.; Dweik, R.A. Single exhaled breath metabolomic analysis identifies unique breathprint in patients with acute decompensated heart failure. J. Am. Coll. Cardiol. 2013, 61, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Cikach, F.S., Jr.; Dweik, R.A. Cardiovascular biomarkers in exhaled breath. Prog. Cardiovasc. Dis. 2012, 55, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Lindstrom, A.B. Collection of a single alveolar exhaled breath for volatile organic compounds analysis. Am. J. Ind. Med. 1995, 28, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.K.; Miekisch, W. Chapter 9 - breath analysis in critically ill patients—potential and limitations. In Volatile biomarkers; Amann, A., Smith, D., Eds.; Elsevier: Boston, 2013; pp. 155–176. [Google Scholar]
- Miekisch, W.; Schubert, J. From highly sophisticated analytical techniques to life-saving diagnostics: Technical developments in breath analysis. TrAC, Trends Anal. Chem. 2006, 25, 665–673. [Google Scholar] [CrossRef]
- Miekisch, W.; Kischkel, S.; Sawacki, A.; Liebau, T.; Mieth, M.; Schubert, J.K. Impact of sampling procedures on the results of breath analysis. J. Breath Res. 2008, 2, 026007. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Kesy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of tedlar(r) bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Birgitta, S.; Fredrik, W.; Anders, V. Analysis of breath samples for lung cancer survival. Anal. Chim. Acta 2014, 840, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Trabue, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of tedlar bags in the measurement of agricultural odorants names are necessary to report factually on available data; however, the usda neither guarantees nor warrants the standard of the product, and use of the name by the usda implies no approval of the product to the exclusion of others that may be suitable. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Mieth, M.; Kischkel, S.; Schubert, J.K.; Hein, D.; Miekisch, W. Multibed needle trap devices for on site sampling and preconcentration of volatile breath biomarkers. Anal. Chem. 2009, 81, 5851–5857. [Google Scholar] [CrossRef] [PubMed]
- Hyšpler, R.; Crhová, Š.; Gasparič, J.; Zadák, Z.; Čı́žková, M.; Balasová, V. Determination of isoprene in human expired breath using solid-phase microextraction and gas chromatography—mass spectrometry. J. Chromatogr. B 2000, 739, 183–190. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Sliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; Kim, K.-H. A review of sampling and pretreatment techniques for the collection of airborne amines. TrAC, Trends Anal. Chem. 2014, 57, 118–134. [Google Scholar] [CrossRef]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Garey, K.W.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Collection and analysis of exhaled breath condensate in humans. Am. J. Respir. Crit. Care. Med. 2001, 164, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzai, H.; Huang, S.; Hettiarachchi, R.; Lin, J.L.; Thomas, P.S.; Zhang, Q. Exhaled breath condensate: A comprehensive update. Clin. Chem. Lab. Med. 2013, 51, 1343–1361. [Google Scholar] [CrossRef] [PubMed]
- Kuban, P.; Foret, F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Anal. Chim. Acta 2013, 805, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rosias, P. Methodological aspects of exhaled breath condensate collection and analysis. J. Breath Res. 2012, 6, 027102. [Google Scholar] [CrossRef] [PubMed]
- Soyer, O.U.; Dizdar, E.A.; Keskin, O.; Lilly, C.; Kalayci, O. Comparison of two methods for exhaled breath condensate collection. Allergy 2006, 61, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Huttmann, E.M.; Greulich, T.; Hattesohl, A.; Schmid, S.; Noeske, S.; Herr, C.; John, G.; Jorres, R.A.; Muller, B.; Vogelmeier, C.; et al. Comparison of two devices and two breathing patterns for exhaled breath condensate sampling. PLoS One 2011, 6, e27467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air part 1: Sorbent-based air monitoring options. J. Chromatogr. A 2010, 1217, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Trimble, T.A.; You, J.; Lydy, M.J. Bioavailability of pcbs from field-collected sediments: Application of tenax extraction and matrix-spme techniques. Chemosphere 2008, 71, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Arce, L.; Valcarcel, M. Enhancing sensitivity and selectivity in the determination of aldehydes in olive oil by use of a tenax ta trap coupled to a uv-ion mobility spectrometer. J. Chromatogr. A 2011, 1218, 7543–7549. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Jimoh, M.A.; Guallar-Hoyas, C.; Creaser, C.S.; Siddiqui, S.; Paul Thomas, C.L. Analysis of human breath samples using a modified thermal desorption: Gas chromatography electrospray ionization interface. J. Breath Res. 2014, 8, 037105. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Blackburn, G.J.; Guallar-Hoyas, C.; Moll, V.H.; Bocos-Bintintan, V.; Kaur-Atwal, G.; Howdle, M.D.; Harry, E.L.; Brown, L.J.; Creaser, C.S.; et al. Detection of volatile organic compounds in breath using thermal desorption electrospray ionization-ion mobility-mass spectrometry. Anal. Chem. 2010, 82, 2139–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wzorek, B.; Mochalski, P.; Sliwka, I.; Amann, A. Application of gc-ms with a spme and thermal desorption technique for determination of dimethylamine and trimethylamine in gaseous samples for medical diagnostic purposes. J. Breath Res. 2010. [Google Scholar] [CrossRef]
- van der Schee, M.P.; Fens, N.; Brinkman, P.; Bos, L.D.; Angelo, M.D.; Nijsen, T.M.; Raabe, R.; Knobel, H.H.; Vink, T.J.; Sterk, P.J. Effect of transportation and storage using sorbent tubes of exhaled breath samples on diagnostic accuracy of electronic nose analysis. J. Breath Res. 2013. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Van Langenhove, H. Sample preparation for the analysis of volatile organic compounds in air and water matrices. J. Chromatogr. A 2007, 1153, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Applications of Solid Phase Microextraction; Royal Society of Chemistry: Cambridge, UK, 1999; Volume 5. [Google Scholar]
- Pawliszyn, J.; Pawliszyn, B.; Pawliszyn, M. Solid phase microextraction (spme). Chem. Educ. 1997, 2, 1–7. [Google Scholar] [CrossRef]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namiesnik, J. Current trends in solid-phase microextraction (spme) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J.; Pedersen-Bjergaard, S. Analytical microextraction: Current status and future trends. J. Chromatogr. Sci. 2006, 44, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Silva, C.L.; Perestrelo, R.; Goncalves, J.; Alves, V.; Camara, J.S. Re-exploring the high-throughput potential of microextraction techniques, spme and meps, as powerful strategies for medical diagnostic purposes. Innovative approaches, recent applications and future trends. Anal. Bioanal. Chem. 2014, 406, 2101–2122. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D.; Zhang, X.; Cudjoe, E.; Pawliszyn, J. Solid-phase microextraction in bioanalysis: New devices and directions. J. Chromatogr. A 2010, 1217, 4041–4060. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Rösner, L.; Hein, D.; Schubert, J.; Miekisch, W. Evaluation of needle trap micro-extraction and automatic alveolar sampling for point-of-care breath analysis. Anal. Bioanal. Chem. 2013, 405, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Lord, H.; Zhan, W.; Pawliszyn, J. Fundamentals and applications of needle trap devices: A critical review. Anal. Chim. Acta 2010, 677, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Kischkel, S.; Hein, D.; James, E.; Schubert, J.; Miekisch, W. Needle trap micro-extraction for voc analysis: Effects of packing materials and desorption parameters. J. Chromatogr. A 2012, 1219, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mesarchaki, E.; Yassaa, N.; Hein, D.; Lutterbeck, H.E.; Zindler, C.; Williams, J. A novel method for the measurement of vocs in seawater using needle trap devices and gc–ms. Mar. Chem. 2014, 159, 1–8. [Google Scholar] [CrossRef]
- Smith, D.; Spaněl, P.; Herbig, J.; Beauchamp, J. Mass spectrometry for real-time quantitative breath analysis. J. Breath Res. 2014, 8, 27101. [Google Scholar] [CrossRef]
- Fink, T.; Baumbach, J.I.; Kreuer, S. Ion mobility spectrometry in breath research. J. Breath Res. 2014, 8, 027104. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sahay, P. Breath analysis using laser spectroscopic techniques: Breath biomarkers, spectral fingerprints, and detection limits. Sensors 2009, 9, 8230–8262. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Jansson, B.O.; Larsson, B.T. Analysis of organic compounds in human breath by gas chromatography-mass spectrometry. J. Lab. Clin. Med. 1969, 74, 961–966. [Google Scholar] [PubMed]
- Chen, S.; Zieve, L.; Mahadevan, V. Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J. Lab. Clin. Med. 1970, 75, 628–635. [Google Scholar] [PubMed]
- Riely, C.A.; Cohen, G.; Lieberman, M. Ethane evolution: A new index of lipid peroxidation. Science 1974, 183, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Dannecker, J.R., Jr.; Shaskan, E.G.; Phillips, M. A new highly sensitive assay for breath acetaldehyde: Detection of endogenous levels in humans. Anal. Biochem. 1981, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Duan, Y. Current status of methods and techniques for breath analysis. Crit. Rev. Anal. Chem. 2007, 37, 3–13. [Google Scholar] [CrossRef]
- Peled, N.; Hakim, M.; Bunn, P.A., Jr.; Miller, Y.E.; Kennedy, T.C.; Mattei, J.; Mitchell, J.D.; Hirsch, F.R.; Haick, H. Non-invasive breath analysis of pulmonary nodules. J. Thorac. Oncol. 2012, 7, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6. [Google Scholar] [CrossRef] [Green Version]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Gastl, G.A.; Amann, A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Elevation of exhaled ethane concentration in asthma. Am. J. Respir. Crit. Care. Med. 2000, 162, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects--confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D'Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D'Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A study of an electronic nose for detection of lung cancer based on a virtual saw gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care. Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Hammel, J.; Dweik, R.; Na, J.; Czich, C.; Laskowski, D.; Mekhail, T. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007, 62, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Annema, J.T.; Schot, R.; van der Schee, M.P.C.; Spanevello, A.; Carratú, P.; Resta, O.; Rabe, K.F.; Sterk, P.J. An electronic nose in the discrimination of patients with non-small cell lung cancer and copd. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, J.W.; Suslick, K.S.; Sasidhar, M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomed. 2013, 9, 15–21. [Google Scholar] [CrossRef]
- Chapman, E.A.; Thomas, P.S.; Stone, E.; Lewis, C.; Yates, D.H. A breath test for malignant mesothelioma using an electronic nose. Eur. Respir. J. 2012, 40, 448–454. [Google Scholar] [CrossRef] [PubMed]
- de Gennaro, G.; Dragonieri, S.; Longobardi, F.; Musti, M.; Stallone, G.; Trizio, L.; Tutino, M. Chemical characterization of exhaled breath to differentiate between patients with malignant plueral mesothelioma from subjects with similar professional asbestos exposure. Anal. Bioanal. Chem. 2010, 398, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; van der Schee, M.P.; Massaro, T.; Schiavulli, N.; Brinkman, P.; Pinca, A.; Carratú, P.; Spanevello, A.; Resta, O.; Musti, M.; et al. An electronic nose distinguishes exhaled breath of patients with malignant pleural mesothelioma from controls. Lung Cancer 2012, 75, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.; Ditkoff, B.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.; Rahbari-Oskoui, F.; Wong, C. Volatile markers of breast cancer in the breath. Breast J. 2003, 9, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436c, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Beatty, J.D.; Cataneo, R.N.; Huston, J.; Kaplan, P.D.; Lalisang, R.I.; Lambin, P.; Lobbes, M.B.I.; Mundada, M.; Pappas, N.; et al. Rapid point-of-care breath test for biomarkers of breast cancer and abnormal mammograms. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Shuster, G.; Gallimidi, Z.; Reiss, A.H.; Dovgolevsky, E.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Engel, A.; Shiban, A.; Tisch, U.; et al. Classification of breast cancer precursors through exhaled breath. Breast Cancer Res. Treat. 2011, 126, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Leunis, N.; Boumans, M.L.; Kremer, B.; Din, S.; Stobberingh, E.; Kessels, A.G.; Kross, K.W. Application of an electronic nose in the diagnosis of head and neck cancer. Laryngoscope 2014, 124, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Ding, L.; Liu, B.B.; Tisch, U.; Xu, Z.Q.; Shi, D.Y.; Zhao, Y.; Chen, J.; Sun, R.X.; Liu, H.; et al. The scent fingerprint of hepatocarcinoma: In-vitro metastasis prediction with volatile organic compounds (VOCs). Int. J. Nanomed. 2012, 7, 4135–4146. [Google Scholar]
- Xue, R.; Dong, L.; Zhang, S.; Deng, C.; Liu, T.; Wang, J.; Shen, X. Investigation of volatile biomarkers in liver cancer blood using solid-phase microextraction and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, H.; Song, Q.; Song, G.; Wang, H.Z.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; Sun, G.P.; Chen, Z.D. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, D.; Li, N.; Zhang, L.; Yang, J. A novel breath analysis system based on electronic olfaction. IEEE Trans. Bio-Med. Eng. 2010. [Google Scholar] [CrossRef]
- Dragonieri, S.; Schot, R.; Mertens, B.J.A.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, M.; Perestrelo, R.; Barros, A.S.; Bilelo, M.J.; Morête, A.; Câmara, J.S.; Rocha, S.M. Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1254, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dallinga, J.W.; Robroeks, C.M.H.H.T.; Van Berkel, J.J.B.N.; Moonen, E.J.C.; Godschalk, R.W.L.; Jöbsis, Q.; Dompeling, E.; Wouters, E.F.M.; Van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2010, 40, 68–76. [Google Scholar] [PubMed]
- Montuschi, P.; Santonico, M.; Mondino, C.; Pennazza, G.; Mantini, G.; Martinelli, E.; Capuano, R.; Ciabattoni, G.; Paolesse, R.; Di Natale, C.; et al. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest 2010, 137, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; van de Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; van Schayck, O.C.P.; Dompeling, E.; van Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.; Weda, H.; Wang, Y.; Knobel, H.H.; Nijsen, T.M.; Vink, T.J.; Zwinderman, A.H.; Sterk, P.J.; Schultz, M.J. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur. Respir. J. 2014. [Google Scholar]
- Schubert, J.K.; Müller, W.P.; Benzing, A.; Geiger, K. Application of a new method for analysis of exhaled gas in critically ill patients. Intensiv. Care Med. 1998, 24, 415–421. [Google Scholar] [CrossRef]
- Fens, N.; Douma, R.A.; Sterk, P.J.; Kamphuisen, P.W. Breathomics as a diagnostic tool for pulmonary embolism. J. Thromb. Haemost. 2010, 8, 2831–2833. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Condos, R.; Ring Erickson, G.A.; Greenberg, J.; La Bombardi, V.; Munawar, M.I.; Tietje, O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 2007, 87, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Basa-Dalay, V.; Bothamley, G.; Cataneo, R.N.; Lam, P.K.; Natividad, M.P.; Schmitt, P.; Wai, J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb.) 2010, 90, 145–151. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Blais, J.; Bothamley, G.; Chaturvedi, A.; Modi, K.D.; Pandya, M.; Natividad, M.P.; Patel, U.; Ramraje, N.N.; et al. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb.) 2012, 92, 314–320. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2000, 162, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, S.M.; Gietema, H.A.; Blanchet, L.; Kruitwagen, C.L.; Munnik, P.; van Klaveren, R.J.; Lammers, J.W.; Buydens, L.; Harren, F.J.; Zanen, P. Screening for emphysema via exhaled volatile organic compounds. J. Breath Res. 2011, 5. [Google Scholar] [CrossRef]
- Hattesohl, A.D.M.; Jörres, R.a.; Dressel, H.; Schmid, S.; Vogelmeier, C.; Greulich, T.; Noeske, S.; Bals, R.; Koczulla, A.R. Discrimination between copd patients with and without alpha 1-antitrypsin deficiency using an electronic nose. Respirology (Carlton, Vic.) 2011, 16, 1258–1264. [Google Scholar] [CrossRef]
- Hauschild, A.C.; Baumbach, J.I.; Baumbach, J. Integrated statistical learning of metabolic ion mobility spectrometry profiles for pulmonary disease identification. Genet. Mol. Res. 2012, 11, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Hengst, M.; Schmid, J.; Buers, H.J.; Mittermaier, B.; Klemp, D.; Koppmann, R. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur. Respir. J. 2006, 27, 929–936. [Google Scholar] [PubMed]
- Kamboures, M.A.; Blake, D.R.; Cooper, D.M.; Newcomb, R.L.; Barker, M.; Larson, J.K.; Meinardi, S.; Nussbaum, E.; Rowland, F.S. Breath sulfides and pulmonary function in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2005, 102, 15762–15767. [Google Scholar] [CrossRef] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Shah, P.L.; Cramer, D.; Hodson, M.E.; Barnes, P.J. Exhaled ethane is elevated in cystic fibrosis and correlates with carbon monoxide levels and airway obstruction. Am. J. Respir. Crit. Care. Med. 2000, 161, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Marcondes-Braga, F.G.; Gutz, I.G.; Batista, G.L.; Saldiva, P.H.; Ayub-Ferreira, S.M.; Issa, V.S.; Mangini, S.; Bocchi, E.A.; Bacal, F. Exhaled acetone as a new biomaker of heart failure severity. Chest 2012, 142, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Duan, J.; Duan, Y. Recent developments of proton-transfer reaction mass spectrometry (PTR-MS) and its applications in medical research. Mass Spectrom. Rev. 2013, 32, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Cikach, F.; Eng, K.; Moses, J.; Patel, N.; Yan, C.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur. J. Gastroenterol. Hepatol. 2014, 26, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; Patel, M.; Pitts, D.; Knight, P.; Hoashi, S.; Evans, M.; Turner, C. The use of a portable breath analysis device in monitoring type 1 diabetes patients in a hypoglycaemic clamp: Validation with sift-ms data. J. Breath Res. 2014. [Google Scholar] [CrossRef]
- Storer, M.; Dummer, J.; Lunt, H.; Scotter, J.; McCartin, F.; Cook, J.; Swanney, M.; Kendall, D.; Logan, F.; Epton, M. Measurement of breath acetone concentrations by selected ion flow tube mass spectrometry in type 2 diabetes. J. Breath Res. 2011, 5, 046011. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of potential biomarkers in exhaled breath for diagnosis of type 2 diabetes mellitus based on gc-ms with metabolomics. RSC Advances 2014, 4, 25430. [Google Scholar] [CrossRef]
- Matsumoto, A.; Hirata, Y.; Kakoki, M.; Nagata, D.; Momomura, S.; Sugimoto, T.; Tagawa, H.; Omata, M. Increased excretion of nitric oxide in exhaled air of patients with chronic renal failure. Clin. Sci. 1999, 96, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pagonas, N.; Vautz, W.; Seifert, L.; Slodzinski, R.; Jankowski, J.; Zidek, W.; Westhoff, T.H. Volatile organic compounds in uremia. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, A.; Smolinska, A.; Dallinga, J.; Masclee, A.; Jonkers, D.; Pierik, M.; van Schooten, F-J. Volatile organic compound in breath as a new test for crohn’s disease activity. United Eur. Gastroenterol. J. 2013, 1, A36. [Google Scholar]
- Crosson, E.R.; Ricci, K.N.; Richman, B.A.; Chilese, F.C.; Owano, T.G.; Provencal, R.A.; Todd, M.W.; Glasser, J.; Kachanov, A.A.; Paldus, B.A.; et al. Stable isotope ratios using cavity ring-down spectroscopy: Determination of 13c/12c for carbon dioxide in human breath. Anal. Chem. 2002, 74, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M. Increased breath ethane and pentane concentrations in currently unmedicated patients with schizophrenia. Open J. Psychiatry 2011, 01, 1–7. [Google Scholar] [CrossRef]
- Herbig, J.; Muller, M.; Schallhart, S.; Titzmann, T.; Graus, M.; Hansel, A. On-line breath analysis with ptr-tof. J. Breath Res. 2009. [Google Scholar] [CrossRef]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Phillip, T.; Markus, S.; Peter, O.; Juliane, O.; Beate, B.; Svend, K.; Jürgen, D.; Ralf, Z.; Jochen, K.S.; Wolfram, M. Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. Anal. Chem. 2013, 85, 10321–10329. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, M.R.; Bakhirkin, Y.; Wysocki, G.; Lewicki, R.; Tittel, F.K. Recent advances of laser-spectroscopy-based techniques for applications in breath analysis. J. Breath Res. 2007. [Google Scholar] [CrossRef]
- Ramgir, N.S. Electronic nose based on nanomaterials: Issues, challenges, and prospects. ISRN Nanomat. 2013, 2013, 21. [Google Scholar] [CrossRef]
- Manginell, R.P.; Bauer, J.M.; Moorman, M.W.; Sanchez, L.J.; Anderson, J.M.; Whiting, J.J.; Porter, D.A.; Copic, D.; Achyuthan, K.E. A monolithically-integrated mugc chemical sensor system. Sensors 2011, 11, 6517–6532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Seo, J.H.; Li, Y.; Chen, D.; Kurabayashi, K.; Fan, X. Smart multi-channel two-dimensional micro-gas chromatography for rapid workplace hazardous volatile organic compounds measurement. Lab. Chip 2013, 13, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Cui, D.F.; Chen, X.; Zhang, L.L.; Cai, H.Y.; Li, H. A micro gas chromatography column with a micro thermal conductivity detector for volatile organic compound analysis. Rev. Sci. Instrum. 2013. [Google Scholar] [CrossRef]
- Fung, A.O.; Mykhaylova, N. Analysis of airborne biomarkers for point-of-care diagnostics. J. Lab. Autom. 2014, 19, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis - focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, E.; Allard, J.P. Breath alkanes as a marker of oxidative stress in different clinical conditions. Free Radic. Biol. Med. 2000, 28, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Kneepkens, C.M.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar] [CrossRef] [PubMed]
- Allerheiligen, S.R.; Ludden, T.M.; Burk, R.F. The pharmacokinetics of pentane, a by-product of lipid peroxidation. Drug Metab. Dispos. 1987, 15, 794–800. [Google Scholar] [PubMed]
- Dryahina, K.; Spanel, P.; Pospisilova, V.; Sovova, K.; Hrdlicka, L.; Machkova, N.; Lukas, M.; Smith, D. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Risby, T.H.; Sehnert, S.S. Clinical application of breath biomarkers of oxidative stress status. Free Radic. Biol. Med. 1999, 27, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Kohlmuller, D.; Kochen, W. Is n-pentane really an index of lipid peroxidation in humans and animals? A methodological reevaluation. Anal. Biochem. 1993, 210, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Gelmont, D.; Stein, R.A.; Mead, J.F. Isoprene-the main hydrocarbon in human breath. Biochem. Biophys. Res. Commun. 1981, 99, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of breath isoprene in healthy volunteers using selected ion flow tube mass spectrometry (SIFT-MS). Physiol. Meas. 2006, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kushch, I.; Arendacka, B.; Stolc, S.; Mochalski, P.; Filipiak, W.; Schwarz, K.; Schwentner, L.; Schmid, A.; Dzien, A.; Lechleitner, M.; et al. Breath isoprene—aspects of normal physiology related to age, gender and cholesterol profile as determined in a proton transfer reaction mass spectrometry study. Clin. Chem. Lab. Med. 2008, 46, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Spanel, P.; Davies, S.; Smith, D. Quantification of breath isoprene using the selected ion flow tube mass spectrometric analytical method. Rapid Commun. Mass Spectrom. 1999, 13, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.P.; Camp, B.J.; Welch, B.E. Trace composition of human respiratory gas. Arch. Environ. Health 1975, 30, 290–295. [Google Scholar] [CrossRef] [PubMed]
- DeMaster, E.G.; Nagasawa, H.T. Isoprene, an endogenous constituent of human alveolar air with a diurnal pattern of excretion. Life Sci. 1978, 22, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Deneris, E.S.; Stein, R.A.; Mead, J.F. In vitro biosynthesis of isoprene from mevalonate utilizing a rat liver cytosolic fraction. Biochem. Biophys. Res. Commun. 1984, 123, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Deneris, E.S.; Stein, R.A.; Mead, J.F. Acid-catalyzed formation of isoprene from a mevalonate-derived product using a rat liver cytosolic fraction. J. Biol. Chem. 1985, 260, 1382–1385. [Google Scholar] [PubMed]
- Mendis, S.; Sobotka, P.A.; Euler, D.E. Pentane and isoprene in expired air from humans: Gas-chromatographic analysis of single breath. Clin. Chem. 1994, 40, 1485–1488. [Google Scholar] [PubMed]
- Jones, A.W.; Lagesson, V.; Tagesson, C. Origins of breath isoprene. J. Clin. Pathol. 1995, 48, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Larstad, M.A.; Toren, K.; Bake, B.; Olin, A.C. Determination of ethane, pentane and isoprene in exhaled air--effects of breath-holding, flow rate and purified air. Acta Physiol. (Oxf) 2007, 189, 87–98. [Google Scholar] [CrossRef]
- Stein, R.A.; Mead, J.F. Small hydrocarbons formed by the peroxidation of squalene. Chem. Phys. Lipids 1988, 46, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Schoeller, D.A. Evidence for diurnal periodicity in human cholesterol synthesis. J. Lipid Res. 1990, 31, 667–673. [Google Scholar] [PubMed]
- Stone, B.G.; Besse, T.J.; Duane, W.C.; Evans, C.D.; DeMaster, E.G. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men. Lipids 1993, 28, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.J. Some new aspects of isoprenoid biosynthesis in plants—a review. Lipids 1995, 30, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Silver, G.M.; Fall, R. Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J. Biol. Chem. 1995, 270, 13010–13016. [Google Scholar] [CrossRef] [PubMed]
- Hyspler, R.; Crhova, S.; Gasparic, J.; Zadak, Z.; Cizkova, M.; Balasova, V. Determination of isoprene in human expired breath using solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. B 2000, 739, 183–190. [Google Scholar] [CrossRef]
- Beytia, E.D.; Porter, J.W. Biochemistry of polyisoprenoid biosynthesis. Annu. Rev. Biochem. 1976, 45, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Connor, W.E. The long term effects of dietary cholesterol upon the plasma lipids, lipoproteins, cholesterol absorption, and the sterol balance in man: The demonstration of feedback inhibition of cholesterol biosynthesis and increased bile acid excretion. J. Lipid Res. 1980, 21, 1042–1052. [Google Scholar] [PubMed]
- Quintao, E.; Grundy, S.M.; Ahrens, E.H., Jr. Effects of dietary cholesterol on the regulation of total body cholesterol in man. J. Lipid Res. 1971, 12, 233–247. [Google Scholar] [PubMed]
- King, J.; Kupferthaler, A.; Frauscher, B.; Hackner, H.; Unterkofler, K.; Teschl, G.; Hinterhuber, H.; Amann, A.; Högl, B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012, 33, 413. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of exhaled breath for disease detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef]
- Fuchs, D.; Jamnig, H.; Heininger, P.; Klieber, M.; Schroecksnadel, S.; Fiegl, M.; Hackl, M.; Denz, H.; Amann, A. Decline of exhaled isoprene in lung cancer patients correlates with immune activation. J. Breath Res. 2012, 6, 027101. [Google Scholar] [CrossRef] [PubMed]
- Rooth, G.; Ostenson, S. Acetone in alveolar air, and the control of diabetes. Lancet 1966, 2, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Trotter, M.D.; Sulway, M.J.; Trotter, E. The rapid determination of acetone in breath and plasma. Clin. Chim. Acta 1971, 35, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A. The diagnostic potential of breath analysis. Clin. Chem. 1983, 29, 5–15. [Google Scholar] [PubMed]
- Hoell, D.; Mensing, T.; Roggenbuck, R.; Sakuth, M.; Sperlich, E.; Urban, T.; Neier, W.; Strehlke, G. 2-Butanone. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Jollivet, N.; Bézenger, M.-C.; Vayssier, Y.; Belin, J.-M. Production of volatile compounds in liquid cultures by six strains of coryneform bacteria. Appl. Microbiol. Biotechnol. 1992, 36, 790–794. [Google Scholar] [CrossRef]
- Ken, W. Volatile metabolites from actinomycetes. Chemosphere 1996, 32, 1427–1434. [Google Scholar] [CrossRef]
- Ron, W.; Christine, H.; Alan, B.; Andrzej, K. Effect of substrate composition on production of volatile organic compounds from trichoderma spp. Inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.-M.M.; Sumner, L.W.; Paré, P.W. Gc-ms spme profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, H.; Tantillo, D.J.; Atsumi, S. Biological production of 2-butanone in escherichia coli. ChemSusChem 2014, 7, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kim, S.; Yum, T.; Kim, Y.; Sang, B.I.; Um, Y. Conversion of levulinic acid to 2-butanone by acetoacetate decarboxylase from clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 2013, 97, 5627–5634. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Razafindrainibe, M.; Drover, J.C. Headspace volatile components of canadian grown low-tannin faba bean (vicia faba l.) genotypes. J. Sci. Food Agric. 2014, 94, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Song, G.C.; Ryu, C.M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Unterkofler, K.; Hinterhuber, H.; Amann, A. Emission rates of selected volatile organic compounds from skin of healthy volunteers. J. Chromatogr. B 2014, 959, 62–70. [Google Scholar] [CrossRef]
- Rudnicka, J.; Mochalski, P.; Agapiou, A.; Statheropoulos, M.; Amann, A.; Buszewski, B. Application of ion mobility spectrometry for the detection of human urine. Anal. Bioanal. Chem. 2010, 398, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8. [Google Scholar] [CrossRef]
- Amann, A.; Costello Bde, L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8. [Google Scholar] [CrossRef]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2012, 50, 573–581. [Google Scholar] [CrossRef]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Shi, D.Y.; Ionescu, R.; Zhang, W.; Hua, Q.L.; Pan, Y.Y.; Tao, L.; Liu, H.; Haick, H. Assessment of ovarian cancer conditions from exhaled breath. Int. J. Cancer 2014. [Google Scholar]
- Ulanowska, A.; Kowalkowski, T.; Hrynkiewicz, K.; Jackowski, M.; Buszewski, B. Determination of volatile organic compounds in human breath for helicobacter pylori detection by SPME-GC/MS. Biomed. Chromatogr. 2011, 25, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9, e1003311. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Belvisi, M.G. Nitric oxide and lung disease. Thorax 1993, 48, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L. Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Ricciardolo, F.L.M. Multiple roles of nitric oxide in the airways. Thorax 2003, 58, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, R.; Gerecitano, J.; Shapiro, D.; Green, S.J.; Aguet, M.; Le, J.; Vilcek, J. Generation of nitric oxide and clearance of interferon-gamma after bcg infection are impaired in mice that lack the interferon-gamma receptor. J. Inflamm. 1995, 46, 23–31. [Google Scholar] [PubMed]
- Green, S.J.; Nacy, C.A.; Schreiber, R.D.; Granger, D.L.; Crawford, R.M.; Meltzer, M.S.; Fortier, A.H. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from l-arginine and protection against francisella tularensis infection in mycobacterium bovis bcg-treated mice. Infect. Immun. 1993, 61, 689–698. [Google Scholar] [PubMed]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular mechanisms of nonspecific immunity to intracellular infection: Cytokine-induced synthesis of toxic nitrogen oxides from l-arginine by macrophages and hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Scheller, L.F.; Marletta, M.A.; Seguin, M.C.; Klotz, F.W.; Slayter, M.; Nelson, B.J.; Nacy, C.A. Nitric oxide: Cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol. Lett. 1994, 43, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Erzurum, S.C. Nitric oxide metabolism in asthma pathophysiology. Biochim. Biophys. Acta 2011, 1810, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Bucca, C.; Cicolin, A.; Guida, G.; Heffler, E.; Brussino, L.; Rolla, G. Exhaled nitric oxide (feno) in non-pulmonary diseases. J. Breath Res. 2012. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ats clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care. Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.F.; Ko, F.W.; Wong, G.W. Recent advances in asthma biomarker research. Ther. Adv. Respir. Dis. 2013, 7, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L. Revisiting the role of exhaled nitric oxide in asthma. Curr. Opin. Pulm. Med. 2014, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ludviksdottir, D.; Diamant, Z.; Alving, K.; Bjermer, L.; Malinovschi, A. Clinical aspects of using exhaled no in asthma diagnosis and management. Clin. Respir. J. 2012, 6, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Ryan, D.; Burden, A.; Von Ziegenweidt, J.; Gould, S.; Freeman, D.; Gruffydd-Jones, K.; Copland, A.; Godley, C.; Chisholm, A.; et al. Using fractional exhaled nitric oxide (feno) to diagnose steroid-responsive disease and guide asthma management in routine care. Clin. Transl. Allergy 2013. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.R. Advances in the clinical applications of exhaled nitric oxide measurements. J. Breath Res. 2012. [Google Scholar] [CrossRef]
- Mandon, J.; Hogman, M.; Merkus, P.J.; van Amsterdam, J.; Harren, F.J.; Cristescu, S.M. Exhaled nitric oxide monitoring by quantum cascade laser: Comparison with chemiluminescent and electrochemical sensors. J. Biomed. Opt. 2012. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.Y.; Kwak, H.J.; Kim, S.I.; Park, D.W.; Kim, J.W.; Kim, T.H.; Sohn, J.W.; Shin, D.H.; Park, S.S.; et al. Comparison of two exhaled nitric oxide analyzers: The niox mino hand-held electrochemical analyzer and the noa280i stationary chemiluminescence analyzer. Respirology 2012, 17, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Hillas, G.; Loukides, S.; Kostikas, K.; Bakakos, P. Biomarkers obtained by non-invasive methods in patients with copd: Where do we stand, what do we expect? Curr. Med. Chem. 2009, 16, 2824–2838. [Google Scholar] [CrossRef] [PubMed]
- Bessa, V.; Tseliou, E.; Bakakos, P.; Loukides, S. Noninvasive evaluation of airway inflammation in asthmatic patients who smoke: Implications for application in clinical practice. Ann. Allergy Asthma Immunol. 2008, 101, 226–232, quiz 232–224, 278. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, H.; Ioannidis, I.; Tomkiewicz, R.P.; de Groot, H.; Rubin, B.K.; Ratjen, F. Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child. 1998, 78, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hogman, M. Extended no analysis in health and disease. J. Breath Res. 2012, 6. [Google Scholar] [CrossRef]
- Fisher, A.J.; Gabbay, E.; Small, T.; Doig, S.; Dark, J.H.; Corris, P.A. Cross sectional study of exhaled nitric oxide levels following lung transplantation. Thorax 1998, 53, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide deficiency in chronic kidney disease. Am. J. Phys. Renal Phys. 2008, 294, F1–F9. [Google Scholar]
- Huang, Y.; Lemberg, D.A.; Day, A.S.; Dixon, B.; Leach, S.; Bujanover, Y.; Jaffe, A.; Thomas, P.S. Markers of inflammation in the breath in paediatric inflammatory bowel disease. J. Pediatr Gastroenterol Nutr. 2014, 59, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, J.; Gu, H.; Zhang, Y.; Pan, S.; Xu, N.; Chen, H.; Li, H. Facilitated diffusion of acetonitrile revealed by quantitative breath analysis using extractive electrospray ionization mass spectrometry. Sci Rep. 2013, 3, 1205. [Google Scholar] [PubMed]
- Greenberg, M. Toxicological review of acetonitrile; U.S. Environmental Protection Agency: Washington, DC, 1999. [Google Scholar]
- Rahman, I.; van Schadewijk, A.A.; Crowther, A.J.; Hiemstra, P.S.; Stolk, J.; MacNee, W.; De Boer, W.I. 4-hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2002, 166, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-p450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol 2008, 4, 697–720. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.D.; Coon, M.J. Hydrocarbon formation in the reductive cleavage of hydroperoxides by cytochrome p-450. Proc. Natl. Acad. Sci. USA. 1987, 84, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.J.; McAdam, K.G.; Winter, D.B.; Liu, C.; Duke, M.G.; Proctor, C.J. Reduction of aldehydes and hydrogen cyanide yields in mainstream cigarette smoke using an amine functionalised ion exchange resin. Chem. Cent. J. 2011. [Google Scholar] [CrossRef]
- Ahotupa, M.; Bussacchini-Griot, V.; Bereziat, J.C.; Camus, A.M.; Bartsch, H. Rapid oxidative stress induced by n-nitrosamines. Biochem. Biophys. Res. Commun. 1987, 146, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Schrader, E.; Hirsch-Ernst, K.I.; Scholz, E.; Kahl, G.F.; Foth, H. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk) in primary cultures of rat alveolar type ii cells. Drug Metab. Dispos. 2000, 28, 180–185. [Google Scholar] [PubMed]
- Hecht, S.S. Recent studies on mechanisms of bioactivation and detoxification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk), a tobacco-specific lung carcinogen. Crit. Rev. Toxicol. 1996, 26, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Akopyan, G.; Bonavida, B. Understanding tobacco smoke carcinogen nnk and lung tumorigenesis. Int. J. Oncol. 2006, 29, 745–752. [Google Scholar] [PubMed]
- Byrne, G.I.; Lehmann, L.K.; Kirschbaum, J.G.; Borden, E.C.; Lee, C.M.; Brown, R.R. Induction of tryptophan degradation in vitro and in vivo: A gamma-interferon-stimulated activity. J. Interferon Res. 1986, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Brandacher, G.; Perathoner, A.; Ladurner, R.; Schneeberger, S.; Obrist, P.; Winkler, C.; Werner, E.R.; Werner-Felmayer, G.; Weiss, H.G.; Gobel, G.; et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating t cells. Clin Cancer Res. 2006, 12, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Brandacher, G.; Winkler, C.; Schroecksnadel, K.; Margreiter, R.; Fuchs, D. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr. Drug Metab. 2006, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, X.; Li, N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography-mass spectrometry. J. Chromatogr. B 2004, 808, 269–277. [Google Scholar] [CrossRef]
- Taivans, I.; Bukovskis, M.; Strazda, G.; Jurka, N. Breath testing as a method for detecting lung cancer. Expert Rev. Anticancer Ther. 2014, 14, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5, S540–s550. [Google Scholar] [PubMed]
- De Boer, N.K.; de Meij, T.G.; Oort, F.A.; Ben Larbi, I.; Mulder, C.J.; van Bodegraven, A.A.; van der Schee, M.P. The scent of colorectal cancer: Detection by volatile organic compound analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Davis, S.D.; Johnson, R.; Esther, C.R., Jr. Exhaled breath condensate purines correlate with lung function in infants and preschoolers. Pediatr Pulmonol 2013, 48, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Marco, S. The need for external validation in machine olfaction: Emphasis on health-related applications. Anal. Bioanal. Chem. 2014, 406, 3941–3956. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.I.; Kell, D.B. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef]
- Smolinska, A.; Hauschild, A.-C.; Fijten, R.; Dallinga, J.; Baumbach, J.; van Schooten, F. Current breathomics-a review on data pre-processing techniques and machine learning in metabolomics breath analysis. J. Breath Res. 2014. [Google Scholar] [CrossRef]
- Miekisch, W.; Herbig, J.; Schubert, J.K. Data interpretation in breath biomarker research: Pitfalls and directions. J. Breath Res. 2012, 6, 036007. [Google Scholar] [CrossRef] [PubMed]
- Malley, J.D.; Dasgupta, A.; Moore, J.H. The limits of p-values for biological data mining. BioData Mining 2013. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Jia, G.; Shah, Z.K.; Pohar, K.; Mortazavi, A.; Zynger, D.L.; Wei, L.; Yang, X.; Clark, D.; Knopp, M.V. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J. Magn. Reson. Imag. 2014. [Google Scholar] [CrossRef]
- Dutta, R.; Kashwan, K.R.; Bhuyan, M.; Hines, E.L.; Gardner, J.W. Electronic nose based tea quality standardization. Neural Netw. 2003, 16, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Von Luxburg, U. A tutorial on spectral clustering. Statist. Comput. 2007, 17, 395–416. [Google Scholar] [CrossRef]
- Krzanowski, W.J. Principles of Multivariate Analysis: A User's Perspective; (Oxford Statistical Science Series;); Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Boots, A.W.; van Berkel, J.J.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. Pls-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.; Westerhuis, J. Double-check: Validation of diagnostic statistics for pls-da models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.a.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; Velzen, E.J.J.; Duijnhoven, J.P.M.; Dorsten, F.A. Assessment of plsda cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Mahadevan, S.; Shah, S.L.; Marrie, T.J.; Slupsky, C.M. Analysis of metabolomic data using support vector machines. Anal. Chem. 2008, 80, 7562–7570. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; López, A.; Peña-Méndez, E.M.; Vaňhara, P.; Hampl, A.; Havel, J. Artificial neural networks in medical diagnosis. J. Appl. Biomed. 2013, 11, 47–58. [Google Scholar] [CrossRef]
- Taylor, J.; King, R.D.; Altmann, T.; Fiehn, O. Application of metabolomics to plant genotype discrimination using statistics and machine learning. 2002; 18, 241–248. [Google Scholar]
- Smolinska, A.; Blanchet, L.; Coulier, L.; Ampt, K.A.M.; Luider, T.; Hintzen, R.Q.; Wijmenga, S.S.; Buydens, L.M.C. Interpretation and visualization of non-linear data fusion in kernel space: Study on metabolomic characterization of progression of multiple sclerosis. PLoS One 2012, 7, e38163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krooshof, P.W.T.; Üstün, B.; Postma, G.J.; Buydens, L.M.C. Visualization and recovery of the (bio)chemical interesting variables in data analysis with support vector machine classification. Anal. Chem. 2010, 82, 7000–7007. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.E.; Rose-Pehrsson, S.L.; McGill, R.A. A comparison study of chemical sensor array pattern recognition algorithms. Anal. Chim. Acta 1999, 384, 305–317. [Google Scholar] [CrossRef]
- Sung, J.; Wang, Y.; Chandrasekaran, S.; Witten, D.M.; Price, N.D. Molecular signatures from omics data: From chaos to consensus. Biotechnol. J. 2012, 7, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- STARD Statement. Available online: http://www.stard-statement.org (accessed on 25 September 2014).
- McShane, L.; Cavenagh, M.; Lively, T.; Eberhard, D.; Bigbee, W.; Williams, P.; Mesirov, J.; Polley, M.-Y.; Kim, K.; Tricoli, J.; et al. Criteria for the use of omics-based predictors in clinical trials: Explanation and elaboration. BMC Med. 2013. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W. Towards complete and accurate reporting of studies of diagnostic accuracy: The stard initiative. Clin. Chem. Lab. Med. 2003, 326, 41–44. [Google Scholar]
- Cunha, M.G.; Hoenigman, S.; Kanchagar, C.; Rearden, P.; Sassetti, C.S.; Trevejo, J.M.; Keshava, N. Joint analysis of differential mobility spectrometer and mass spectrometer features for tuberculosis biomarkers. Conf Proc. IEEE Eng. Med. Biol Soc. 2008, 2008, 359–362. [Google Scholar] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. Hmdb: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Halling-Brown, M.D.; Bulusu, K.C.; Patel, M.; Tym, J.E.; Al-Lazikani, B. Cansar: An integrated cancer public translational research and drug discovery resource. Nucleic Acids Res. 2012, 40, D947–D956. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview. Metabolites 2015, 5, 3-55. https://doi.org/10.3390/metabo5010003
Pereira J, Porto-Figueira P, Cavaco C, Taunk K, Rapole S, Dhakne R, Nagarajaram H, Câmara JS. Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview. Metabolites. 2015; 5(1):3-55. https://doi.org/10.3390/metabo5010003
Chicago/Turabian StylePereira, Jorge, Priscilla Porto-Figueira, Carina Cavaco, Khushman Taunk, Srikanth Rapole, Rahul Dhakne, Hampapathalu Nagarajaram, and José S. Câmara. 2015. "Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview" Metabolites 5, no. 1: 3-55. https://doi.org/10.3390/metabo5010003
APA StylePereira, J., Porto-Figueira, P., Cavaco, C., Taunk, K., Rapole, S., Dhakne, R., Nagarajaram, H., & Câmara, J. S. (2015). Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview. Metabolites, 5(1), 3-55. https://doi.org/10.3390/metabo5010003