Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
2.2. Applicability of the Method
3. Materials and Methods
3.1. Chemicals
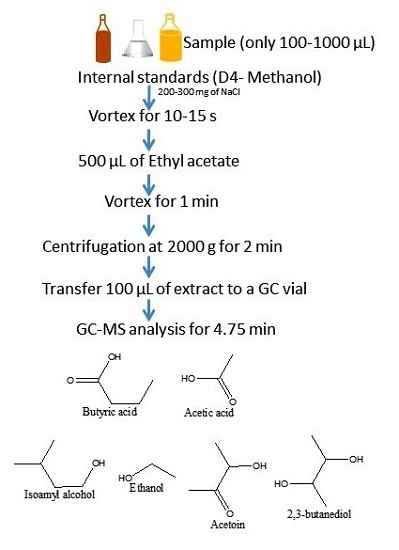
3.2. Sample Preparation
3.3. GC-MS Analysis
3.4. Validation of the Method
3.5. Application of the Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography-mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| CV | Coefficient of variation |
| RSD | Residual standard deviation |
References
- Villas-Boas, S.G.; Mas, S.; Akesson, M.; Smedsgaard, J.; Nielsen, J. Mass spectrometry in metabolome analysis. Mass Spectrom. Rev. 2005, 24, 613–646. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Qian, M.C. Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged chinese “Yanghe Daqu” liquors. J. Agric. Food Chem. 2005, 53, 7931–7938. [Google Scholar] [CrossRef] [PubMed]
- Hayaloglu, A.A.; Karabulut, I. SPME/GC-MS characterization and comparison of volatiles of eleven varieties of turkish cheeses. Int. J. Food Prop. 2013, 16, 1630–1653. [Google Scholar] [CrossRef]
- Pino, J.A.; Roncal, E. Validation of a HS-SPME-GC method for determining higher fatty esters and oak lactones in white rums. Food Anal. Methods 2015, 1–5. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, J.; Wang, L.; Li, Z. Development of a SPME-GC-MS method for the determination of volatile compounds in shanxi aged vinegar and its analytical characterization by aroma wheel. J. Food Sci. Technol. 2016, 53, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2015, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Stackler, B.; Christensen, E.N. Quantitative determination of ethanol in wine by gas chromatography. Am. J. Enol. Vitic. 1974, 25, 202–2007. [Google Scholar]
- Lázaro, F.; de Castro, M.D.L.; Valcárcel, M. Individual and simultaneous enzymatic determination of ethanol and acetaldehyde in wines by flow injection analysis. Anal. Chim. Acta 1986, 185, 57–64. [Google Scholar] [CrossRef]
- Gonchar, M.V.; Maidan, M.M.; Pavlishko, H.M.; Sibirny, A.A. A new oxidase-peroxidase kit for ethanol assays in alcoholic beverages. Food Technol. Biotechnol. 2001, 39, 37–42. [Google Scholar]
- Kupina, S.A. Simultaneous quantitation of glycerol, acetic acid and ethanol in grape juice by high performance liquid chromatography. Am. J. Enol. Vitic. 1984, 35, 59–62. [Google Scholar]
- Christensen, L.M.; Fulmer, E.I. Analysis of n-butanol, acetone, and ethanol in aqueous solution. Ind. Eng. Chem. 1935, 7, 180–182. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of Aoac International; AOAC International: Gaithersburg, MD, USA, 2012; Vol. 19, Available online: http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=BFHIA.xis&method=post&formato=2&cantidad=1&expresion=mfn=016229 (accessed on 26 July 2017).
- Calull, M.; Marce, R.M.; Borrull, F. Determination of carboxylic acids, sugars, glycerol and ethanol in wine and grape must by ion-exchange high-performance liquid chromatography with refractive index detection. J. Chromatogr. 1992, 590, 215–222. [Google Scholar] [CrossRef]
- Zhang, S.M.; Yang, Y.; Ni, Y.Y. Combination of near infrared spectroscopy and electronic nose for alcohol quantification during the red wine fermentation. Guang Pu Xue Yu Guang Pu Fen Xi 2012, 32, 2997–3001. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23387165 (accessed on 26 July 2017). [PubMed]
- Garcia-Jares, C.M.; Médina, B. Application of multivariate calibration to the simultaneous routine determination of ethanol, glycerol, fructose, glucose and total residual sugars in botrytized-grape sweet wines by means of near-infrared reflectance spectroscopy. Fresenius J. Anal. Chem. 1997, 357, 86–91. [Google Scholar] [CrossRef]
- Wang, M.L.; Choong, Y.M.; Su, N.W.; Lee, M.S. A rapid method for determination of ethanol in alcoholic beverages using capillary gas chromatography. J. Food Drug Anal. 2003, 11, 133–140. [Google Scholar]
- Páscoa, R.N.M.J.; Vidigal, S.S.M.P.; Tóth, I.V.; Rangel, A.O.S.S. Sequential injection system for the enzymatic determination of ethanol in wine. J. Agric. Food Chem. 2006, 54, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Falqué López, E.; Fernández Gómez, E. Simultaneous determination of the major organic acids, sugars, glycerol, and ethanol by hplc in grape musts and white wines. J. Chromatogr. Sci. 1996, 34, 254–257. [Google Scholar] [CrossRef]
- Paredes, E.; Maestre, S.E.; Prats, S.; Todolí, J.L. Simultaneous determination of carbohydrates, carboxylic acids, alcohols, and metals in foods by high-performance liquid chromatography inductively coupled plasma atomic emission spectrometry. Anal. Chem. 2006, 78, 6774–6782. [Google Scholar] [CrossRef] [PubMed]
- Gerchman, Y.; Schnitzer, A.; Gal, R.; Mirsky, N.; Chinkov, N. A simple rapid gas-chromatography flame-ionization-detector (GC-FID) method for the determination of ethanol from fermentation processes. Afr. J. Biotechnol. 2012, 11, 3612–3616. [Google Scholar] [CrossRef]
- Wang, M.L.; Wang, J.T.; Choong, Y.M. Simultaneous quantification of methanol and ethanol in alcoholic beverage using a rapid gas chromatographic method coupling with dual internal standards. Food Chem. 2004, 86, 609–615. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. Solid phase extraction, multidimensional gas chromatography mass spectrometry determination of four novel aroma powerful ethyl esters. Assessment of their occurrence and importance in wine and other alcoholic beverages. J. Chromatogr. A 2007, 1140, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, I.A.; Al-Awadhi, A.H.; Al-Hatali, Z.N.; AI-Rayami, F.J.; AI-Katheeri, N.A. Rapid and sensitive static headspace gas chromatography-mass spectrometry method for the analysis of ethanol and abused inhalants in blood. J. Chromatogr. B 2004, 799, 331–336. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Altshuller, A.P.; Everson, H.E. The solubility of ethyl acetate in water. J. Am. Chem. Soc. 1953, 75, 1727. [Google Scholar] [CrossRef]
- Shah, D.J.; Tiwari, K.K. Effect of salt on the distribution of acetic acid between water and organic solvent. J. Chem. Eng. Data 1981, 26, 375–378. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Fugelsang, K.E.; Gump, B.H.; Nury, E.S. Wine Analysis and Production; Chapman & Hall: New York, NY, USA, 1995; Available online: http://www.springer.com/gp/book/9781475769678 (accessed on 26 July 2017).
- Monica Lee, K.Y.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of flavour in whiskies and a revised flavour wheel: A review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P.A. Influence of grape-harvesting steps on varietal th ol aromas in sauvignon blanc wines. J. Agric. Food Chem. 2011, 59, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile compounds from grape skin, juice and wine from five interspecific hybrid grape cultivars grown in Québec (Canada) for wine production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of odorants in varietal wines from international grape cultivars (Vitis vinifera) grown in nw spain. S. Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Schlatter, J.; Chiadmi, F.; Gandon, V.; Chariot, P. Simultaneous determination of methanol, acetaldehyde, acetone, and ethanol in human blood by gas chromatography with flame ionization detection. Hum. Exp. Toxicol. 2014, 33, 74–80. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Retention Time (min) | Reference Ion | Detection Limit (mg/L) | Quantification Limit (mg/L) | Range of Quantification (mg/L) | Regression Line (n = 5) | Coefficient (r2) |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| D4-methanol (Internal standard) | 1.780 | 33 | - | - | - | - | - |
| Methanol | 1.786 | 32 | 5 | 10 | 100–80000 | y = 17.926x − 1.528 | 0.9999 |
| Ethanol* | 1.847 | 31 | 0.5* <0.1+ | 2* <1+ | 1–350000 | y = 4.321x − 1.237* y = 2.129x − 0.987+ | 0.9999* 0.9970+ |
| Isobutanol | 2.280 | 43 | 5 | 10 | 10–50000 | y = 4.219x − 0.8876 | 0.9987 |
| 1-butanol | 2.417 | 56 | 5 | 10 | 10–20000 | y =6.2622x − 0.9914 | 0.9979 |
| Isoamyl alcohol | 2.698 | 55 | 5 | 10 | 10–80000 | y = 9.9944x − 1.41 | 0.9950 |
| 4-methyl pentanol | 2.729 | 69 | 4 | 10 | 10–50000 | y = 10.957x − 1.1913 | 0.9975 |
| cis-3-hexen-1-ol | 3.154 | 41 | 12 | 40 | 40–6000 | y = 4.321x − 0.875 | 0.9931 |
| 2,3-butanediol | 3.204 | 57 | 8 | 20 | 20–10000 | y = 5.218x − 1.102 | 0.9901 |
| trans-3-hexen-1-ol | 3.200 | 67 | 20 | 50 | 50–7000 | y = 2.135x − 0.2981 | 0.9912 |
| 2-pentanol | 3.497 | 45 | 8 | 12 | 12–50000 | y = 20.032x − 0.2981 | 0.9996 |
| 1,3-propandiol | 3.569 | 57 | 10.6 | 25 | 25–10000 | y = 0.0164x + 0.1013 | 0.9968 |
| 1-phenylethyl alcohol | 4.237 | 107 | 10 | 20 | 20–20000 | y = 0.2937x + 7.2903 | 0.9976 |
| 2-phenylethyl alcohol | 4.508 | 91 | 10 | 20 | 20–20000 | y = 0.392x + 8.9852 | 0.9930 |
| Aldehydes and ketones | |||||||
| Acetone | 1.962 | 58 | 3 | 8 | 8–50000 | y = 0.1551x + 0.8478 | 0.9945 |
| Acetoin | 2.721 | 43 | 1 | 6 | 6–20000 | y = 0.1352x + 0.5321 | 0.9951 |
| Hexenal | 2.804 | 56 | 3 | 8 | 8–10000 | y = 1.098x + 0.0251 | 0.9904 |
| 2-hexenal | 3.175 | 43 | 1 | 3 | 3–9000 | y = 2.198x + 0.984 | 0.9913 |
| Butyrolactone | 3.963 | 42 | ND | ND | ND | ND | ND |
| Volatile acids | |||||||
| Acetic acid* | 2.346 | 43 | 0.4* <0.1+ | 1.5* <0.5+ | 1.5–50000 | y = 0.1247x + 0.875* y = 0.0987x + 0.654+ | 0.9956* 0.9942+ |
| Propanoic acid | 2.890 | 74 | 1 | 5 | 5–10000 | y = 0.0756x + 0.5821 | 0.9986 |
| Isobutyric acid | 3.053 | 73 | 1 | 2.5 | 2.5–12000 | y = 0.1429x + 2.3651 | 0.9972 |
| Butyric acid | 3.249 | 60 | 1 | 2 | 2–10000 | y = 0.1049x + 4.2899 | 0.9980 |
| Isovaleric acid | 3.426 | 60 | 1 | 5 | 5–8000 | y = 0.1256x + 1.2098 | 0.9976 |
| Valeric acid | 3.674 | 60 | 0.9 | 2 | 2–9500 | y = 0.1109x + 5.1481 | 0.9993 |
| Hexanoic acid | 4.056 | 60 | 4 | 8 | 8–9500 | y = 0.1142x + 2.6098 | 0.9991 |
| 3-hydroxybutyric acid | 4.281 | 60 | 5 | 10 | 10–10000 | y = 0.0253x + 0.0138 | 0.9990 |
| Heptanoic acid | 4.580 | 73 | 9 | 16 | 16–30000 | y = 0.345x + 4.219 | 0.9870 |
| Octanoic acid | 4.690 | 60 | 8 | 14 | 14–20000 | y = 0.536x + 5.453 | 0.9840 |
| Esters | |||||||
| Ethyl isobutyrate | 2.508 | 43 | 8 | 12 | 12–10000 | y = 0.536x + 5.453 | 0.9912 |
| Isobutyl acetate | 2.600 | 43 | 8 | 12 | 12–10000 | y = 0.536x + 5.453 | 0.9943 |
| Ethyl butanoate | 2.700 | 71 | 8 | 12 | 12–10000 | y = 0.536x + 5.453 | 0.9950 |
| Pyruvic aldehyde dimethyl acetate | 2.901 | 75 | 8 | 12 | 12–10000 | y = 0.536x + 5.453 | 0.9933 |
| Ethyl-L-lactate | 3.030 | 75 | 1 | 5 | 5–20000 | y = 0.0545x + 0.0953 | 0.9985 |
| Isoamyl acetate | 3.102 | 70 | 2 | 4 | 4–20000 | y = 0.0786x + 0.128 | 0.9956 |
| Ethyl caproate | 3.620 | 88 | 8 | 15 | 15–10000 | y = 0.0037x + 0.0058 | 0.9993 |
| Hexyl acetate | 3.706 | 84 | 1 | 5 | 5–15000 | y = 1.235x − 0.986 | 0.9912 |
| Ethyl caprylate | 4.399 | 88 | 4 | 6 | 6–10000 | y = 0.3509x + 3.0032 | 0.9943 |
| Diethyl succinate | 4.580 | 101 | 1 | 5 | 5–10500 | y = 0.4808x + 2.8397 | 0.9958 |
| Diethyl malate | 5.002 | 117 | 1 | 5 | 5–10000 | y = 0.5632x + 1.298 | 0.9987 |
| Others | |||||||
| Linalool | 4.123 | 71 | 5 | 10 | 10-5000 | y = 4.437x − 1.235 | 0.9965 |
| Matrix | Ethanol | Acetic Acid | Ethyl-L-lactate | Isoamyl Alcohol | Acetoin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | ||||||
| RT | RP | RT | RP | RT | RP | RT | RP | RT | RP | |
| Standard solution (Intra-day) | 0.8 | 1.1 | 0.8 | 1.0 | 1.2 | 1.6 | 0.7 | 1.1 | 1.3 | 1.7 |
| Standard solution (Inter-day) | 3.4 | 4.2 | 3.8 | 4.4 | 2.0 | 2.5 | 1.8 | 2.1 | 2.5 | 4.5 |
| Beer | 2.7 | 2.5 | 1.3 | 2.5 | 2.0 | 3.2 | 1.5 | 2.1 | 3.2 | 3.6 |
| Red wine | 1.8 | 2.3 | 2.2 | 2.6 | 3.0 | 3.2 | 2.4 | 4.1 | 2.3 | 3.5 |
| Synthetic wine | 1.1 | 1.6 | 2.0 | 2.6 | 3.1 | 3.3 | 2.1 | 2.6 | 3.3 | 3.9 |
| White wine | 1.5 | 2.1 | 2.5 | 3.0 | 2.8 | 3.6 | 3.2 | 4.5 | 3.5 | 4.6 |
| Whisky | 1.7 | 1.8 | 3.4 | 4.1 | ND | ND | 2.1 | 2.7 | 3.8 | 4.2 |
| Vinegar | 1.2 | 1.3 | 1.3 | 1.6 | 3.4 | 3.9 | 4.1 | 4.5 | 2.2 | 3.5 |
| Matrix | ||||||
|---|---|---|---|---|---|---|
| Standard solution 1 | Standard solution 2 | Spiked synthetic wine | Spiked red wine | Spiked white wine | ||
| Ethanol | Actual concentration (mg/L) | 7.89 | 394.5 | 94680.0 | 149910.0 | 126260.0 |
| Determined concentration (mg/L) | 7.93 ± 0.22 | 394.3 ± 0.76 | 94780.2 ± 340.3 | 151410.2 ± 1200.0 | 126220.3 ± 980.3 | |
| Recovery (%) | 101.51 | 99.95 | 100.10 | 101 | 99.97 | |
| Acetic acid | Actual concentration (mg/L) | 5.05 | 630 .0 | 450.0 | 880 | 1200.0 |
| Determined concentration (mg/L) | 5.07 ± 0.03 | 629.2 ± 9.7 | 447.5 ± 5.8 | 885.9 ± 9.5 | 1208.2 ± 7.4 | |
| Recovery (%) | 100.39 | 99.87 | 99.44 | 100.67 | 100.67 | |
| Ethyl-L-lactate | Actual concentration (mg/L) | 10.50 | 30.45 | 70.25 | 500.60 | 1000.2 |
| Determined concentration (mg/L) | 10.95 ± 0.13 | 30.50 ± 0.25 | 69.88 ± 0.79 | 515.55 ± 20.2 | 1062.8 ± 11.1 | |
| Recovery (%) | 104.28 | 100.16 | 99.47 | 102.98 | 106.25 | |
| Isoamyl alcohol | Actual concentration (mg/L) | 20.5 | 500.0 | 1500.0 | 5250.0 | 2500.5 |
| Determined concentration (mg/L) | 20.9 ± 3.5 | 501.8 ± 10.3 | 1450.3 ± 30.5 | 5215.8 ± 12.9 | 2543.5 ± 50.6 | |
| Recovery (%) | 101.95 | 100.36 | 96.67 | 99.34 | 101.72 | |
| Acetoin | Actual concentration (mg/L) | 12.5 | 50.0 | 250.5 | 1045.0 | 555.0 |
| Determined concentration (mg/L) | 12.6 ± 1.5 | 49.3 ± 0.55 | 251.5 ± 6.5 | 1032.1 ± 22.1 | 561.8 ± 8.1 | |
| Recovery (%) | 100.8 | 98.52 | 100.40 | 98.76 | 101.22 | |
| Metabolite | Concentration In Fermented Food and Beverages (mg/L) | |||||
|---|---|---|---|---|---|---|
| Sourdough (n = 3) | Balsamic vinegars (n = 6) | Beer (n = 3) | Red wine (n = 3) | White wine (n = 3) | Whisky (n = 3) | |
| Ethanol | 1750.85 ± 200.18 | 1900.78 ± 750.47 | 48730.98 ± 6000.99 | 107150.25 ± 5500.51 | 95890.45 ± 8700.64 | 330780.89 ± 90000.64 |
| Acetic acid | 1398.55 ± 65.99 | 5910.62 ± 300.78 | 300.12 ± 20.66 | 455.17 ± 145.10 | 260.18 ± 79.24 | 50.29 ± 32.99 |
| Acetoin | 200.76 ± 45.66 | 1558.87 ± 400.34 | 100.78 ± 45.62 | 240.66 ± 98.24 | 100.42 ± 65.43 | 60.35 ± 10.92 |
| Propanoic acid | 110.45 ± 22.38 | 750.92 ± 100.27 | 150.36 ± 20.99 | 198.26 ± 35.77 | 120.11 ± 39.90 | ND |
| Butyric acid | 200.65 ± 26.21 | 890.45 ± 76.27 | 160.91 ± 10.87 | 155.21 ± 86.10 | 145.78 ± 14.28 | ND |
| 2,3-butanediol | 167.26±15.55 | 300.99 ± 90.81 | 100.24 ± 26.87 | 500.25 ± 155.42 | 321.85 ± 109.26 | 65.12 ± 10.78 |
| cis-3-hexen-1-ol | ND | ND | ND | ULQ | 100.76 ± 80.22 | ND |
| Isoamyl alcohol | ULQ | 240.99 ± 56.71 | 105.50 ± 28.91 | 300.97 ± 109.28 | 230.89 ± 56.20 | 500.98 ± 120.90 |
| 1-butanol | ULQ | 120.98 ± 17.58 | 100.11 ± 9.87 | 110.27 ± 4.78 | ULQ | 200.90 ± 56.72 |
| 1-pentanol | ND | ND | ND | ULQ | ULQ | 145.87 ± 34.99 |
| Phenylethyl alcohol | 100.10 ± 23.98 | 200.34 ± 55.40 | 100.21 ± 45.99 | 450.92 ± 100.21 | 300.65 ± 145.12 | 240.98 ± 48.92 |
| Linalool | ND | ND | ND | ULQ | ULQ | ND |
| Ethyl-L-Lactate | 110.99 ± 9.76 | 230.97 ± 56.78 | 105.67 ± 20.98 | 500.98 ± 200.17 | 300.97 ± 87.22 | ULQ |
| Phenyl ethyl acetate | ULQ | ULQ | ULQ | 140.43 ± 32.88 | 100.35 ± 4.90 | ND |
| Diethyl succinate | ULQ | 150.97 ± 34.55 | 120.97 ± 16.38 | 206.76 ± 23.76 | 130.98 ± 76.12 | 100.24 ± 6.54 |
| Diethyl malate | ULQ | 100.65 ± 54.98 | ULQ | 100.89 ± 23.19 | 120.97 ± 32.90 | ND |
| Ethyl caprylate | ULQ | ULQ | ULQ | 167.98 ± 34.89 | 104.51 ± 4.31 | ND |
| Hexyl acetate | ND | ULQ | ULQ | ULQ | 109.26 ± 3.78 | ND |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinu, F.R.; Villas-boas, S.G. Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry. Metabolites 2017, 7, 37. https://doi.org/10.3390/metabo7030037
Pinu FR, Villas-boas SG. Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry. Metabolites. 2017; 7(3):37. https://doi.org/10.3390/metabo7030037
Chicago/Turabian StylePinu, Farhana R., and Silas G. Villas-boas. 2017. "Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry" Metabolites 7, no. 3: 37. https://doi.org/10.3390/metabo7030037
APA StylePinu, F. R., & Villas-boas, S. G. (2017). Rapid Quantification of Major Volatile Metabolites in Fermented Food and Beverages Using Gas Chromatography-Mass Spectrometry. Metabolites, 7(3), 37. https://doi.org/10.3390/metabo7030037