Annotating Nontargeted LC-HRMS/MS Data with Two Complementary Tandem Mass Spectral Libraries
Abstract
:1. Introduction
2. Results and Discussion
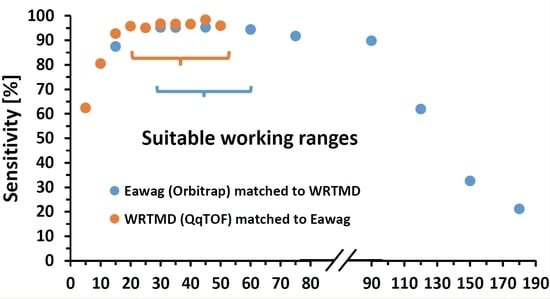
2.1. Testing and Benchmarking of the Tandem Mass Spectral Libraries
2.2. Compound Annotation Workflow for Application Samples
2.3. Application Work
2.3.1. Application 1: Systematic Toxicological Analysis of Human Plasma Samples
2.3.2. Application 2: Comprehensive Compound Identification in Wastewater Influent Samples Collected in a Local Wastewater Treatment Plant (WWTP)
2.3.3. Application 3: Retrospective Compound Identification in LC-MS/MS Data Acquired from Swiss Wastewater Effluent Samples
3. Materials and Methods
3.1. Tandem Mass Spectral Libraries
3.2. Tandem Mass Spectral Library Search
3.3. Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS- and GC/MS-based metabolomics: State of the field and future prospects. TrAC-Trend. Anal. Chem. 2016, 78, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.J.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M.; et al. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. 2018, 37, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Arnhard, K. Current status of non-targeted liquid chromatography-tandem mass spectrometry in forensic toxicology. TrAC-Trend. Anal. Chem. 2016, 84, 94–105. [Google Scholar] [CrossRef]
- Blazenovic, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Arnhard, K. Compound identification in forensic toxicological analysis with untargeted LC-MS-based techniques. Bioanalysis 2015, 7, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Milman, B.L.; Zhurkovich, I.K. Mass spectral libraries: A statistical review of the visible use. TrAC-Trend. Anal. Chem. 2016, 80, 636–640. [Google Scholar] [CrossRef]
- Stein, S. Mass spectral reference libraries: An ever-expanding resource for chemical identification. Anal. Chem. 2012, 84, 7274–7282. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC-high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef]
- Chiaia-Hernandez, A.C.; Schymanski, E.L.; Kumar, P.; Singer, H.P.; Hollender, J. Suspect and nontarget screening approaches to identify organic contaminant records in lake sediments. Anal. Bioanal. Chem. 2014, 406, 7323–7335. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Schymanski, E.L.; Bletsou, A.A.; Aalizadeh, R.; Hollender, J.; Thomaidis, N.S. Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS. Environ. Sci. Technol. 2015, 49, 12333–12341. [Google Scholar] [CrossRef]
- Zedda, M.; Zwiener, C. Is nontarget screening of emerging contaminants by LC-HRMS successful? A plea for compound libraries and computer tools. Anal. Bioanal. Chem. 2012, 403, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Arnhard, K.; Gottschall, A.; Pitterl, F.; Oberacher, H. Applying ‘Sequential Windowed Acquisition of all Theoretical Fragment Ion Mass Spectra’ (SWATH) for systematic toxicological analysis with liquid chromatography-high-resolution tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Schubert, B.; Libiseller, K.; Schweissgut, A. Detection and identification of drugs and toxicants in human body fluids by liquid chromatography-tandem mass spectrometry under data-dependent acquisition control and automated database search. Anal. Chim. Acta 2013, 770, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Pavlic, M.; Libiseller, K.; Schubert, B.; Sulyok, M.; Schuhmacher, R.; Csaszar, E.; Kofeler, H.C. On the inter-instrument and inter-laboratory transferability of a tandem mass spectral reference library: 1. Results of an Austrian multicenter study. J. Mass Spectrom. 2009, 44, 485–493. [Google Scholar] [CrossRef]
- Pavlic, M.; Libiseller, K.; Oberacher, H. Combined use of ESI-QqTOF-MS and ESI-QqTOF-MS/MS with mass-spectral library search for qualitative analysis of drugs. Anal. Bioanal. Chem. 2006, 386, 69–82. [Google Scholar] [CrossRef]
- Stravs, M.A.; Schymanski, E.L.; Singer, H.P.; Hollender, J. Automatic recalibration and processing of tandem mass spectra using formula annotation. J. Mass Spectrom. 2013, 48, 89–99. [Google Scholar] [CrossRef]
- Yang, X.Y.; Neta, P.; Stein, S.E. Quality control for building libraries from electrospray ionization tandem mass spectra. Anal. Chem. 2014, 86, 6393–6400. [Google Scholar] [CrossRef]
- Oberacher, H.; Weinmann, W.; Dresen, S. Quality evaluation of tandem mass spectral libraries. Anal. Bioanal. Chem. 2011, 400, 2641–2648. [Google Scholar] [CrossRef]
- Shahaf, N.; Rogachev, I.; Heinig, U.; Meir, S.; Malitsky, S.; Battat, M.; Wyner, H.; Zheng, S.N.; Wehrens, R.; Aharoni, A. The WEIZMASS spectral library for high-confidence metabolite identification. Nat. Commun. 2016, 7, 12423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. Metlin: A metabolite mass spectral database. Ther. Drug. Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. Massbank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Whitley, G.; Berger, B. Evaluation of the sensitivity of the ‘Wiley Registry of Tandem Mass Spectral Data, MSforID’ with MS/MS data of the ‘NIST/NIH/EPA mass spectral library’. J. Mass Spectrom. 2013, 48, 487–496. [Google Scholar] [CrossRef]
- Oberacher, H.; Pavlic, M.; Libiseller, K.; Schubert, B.; Sulyok, M.; Schuhmacher, R.; Csaszar, E.; Kofeler, H.C. On the inter-instrument and the inter-laboratory transferability of a tandem mass spectral reference library: 2. Optimization and characterization of the search algorithm. J. Mass Spectrom. 2009, 44, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, R.; Mauron, Y.; Masselot, A.; Binz, P.A.; Budin, N.; Fathi, M.; Viette, V.; Hochstrasser, D.F.; Lisacek, F. X-Rank: A robust algorithm for small molecule identification using tandem mass spectrometry. Anal. Chem. 2009, 81, 7604–7610. [Google Scholar] [CrossRef]
- Oberacher, H.; Whitley, G.; Berger, B.; Weinmann, W. Testing an alternative search algorithm for compound identification with the ‘Wiley Registry of Tandem Mass Spectral Data, MSforID’. J. Mass Spectrom. 2013, 48, 497–504. [Google Scholar] [CrossRef]
- Frainay, C.; Schymanski, E.L.; Neumann, S.; Merlet, B.; Salek, R.M.; Jourdan, F.; Yanes, O. Mind the gap: Mapping mass spectral databases in genome-scale metabolic networks reveals poorly covered areas. Metabolites 2018, 8, 51. [Google Scholar] [CrossRef]
- Oberacher, H.; Pitterl, F.; Siapi, E.; Steele, B.R.; Letzel, T.; Grosse, S.; Poschner, B.; Tagliaro, F.; Gottardo, R.; Chacko, S.A.; et al. On the inter-instrument and the inter-laboratory transferability of a tandem mass spectral reference library. 3. Focus on ion trap and upfront CID. J. Mass Spectrom. 2012, 47, 263–270. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Singer, H.P.; Longree, P.; Loos, M.; Ruff, M.; Stravs, M.A.; Vidal, C.R.; Hollender, J. Strategies to characterize polar organic contamination in wastewater: Exploring the capability of high resolution mass spectrometry. Environ. Sci. Technol. 2014, 48, 1811–1818. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Singer, H.P.; Slobodnik, J.; Ipolyi, I.M.; Oswald, P.; Krauss, M.; Schulze, T.; Haglund, P.; Letzel, T.; Grosse, S.; et al. Non-target screening with high-resolution mass spectrometry: Critical review using a collaborative trial on water analysis. Anal. Bioanal. Chem. 2015, 407, 6237–6255. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Austin, C.; Patel, D.; Dolios, G.; Awawda, M.; Arora, M. Trends in the application of high-resolution mass spectrometry for human biomonitoring: An analytical primer to studying the environmental chemical space of the human exposome. Environ. Int. 2017, 100, 32–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitterl, F.; Kob, S.; Pitterle, J.; Steger, J.; Oberacher, H. Applying LC with low resolution MS/MS and subsequent library search for reliable compound identification in systematic toxicological analysis. LC GC Eur. 2016, 29, 419–427. [Google Scholar]
- Steger, J.; Arnhard, K.; Haslacher, S.; Geiger, K.; Singer, K.; Schlapp, M.; Pitterl, F.; Oberacher, H. Successful adaption of a forensic toxicological screening workflow employing nontargeted liquid chromatography-tandem mass spectrometry to water analysis. Electrophoresis 2016, 37, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, Y.; Guo, Y.; Chen, F.; Zhu, Z.-J. MetDIA: Targeted metabolite extraction of multiplexed MS/MS spectra generated by data-independent acquisition. Anal. Chem. 2016, 88, 8757–8764. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Samanipour, S.; Hollender, J.; Ibanez, M.; Kaserzon, S.; Kokkali, V.; van Leerdam, J.A.; Mueller, J.F.; Pijnappels, M.; Reid, M.J.; et al. Exploring the potential of a global emerging contaminant early warning network through the use of retrospective suspect screening with high-resolution mass spectrometry. Environ. Sci. Technol. 2018, 52, 5135–5144. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H. The Wiley Registry of Tandem Mass Spectral Data, MSforID, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-03744-7. [Google Scholar]
- Kessner, D.; Chambers, M.; Burke, R.; Agusand, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberacher, H.; Reinstadler, V.; Kreidl, M.; Stravs, M.A.; Hollender, J.; Schymanski, E.L. Annotating Nontargeted LC-HRMS/MS Data with Two Complementary Tandem Mass Spectral Libraries. Metabolites 2019, 9, 3. https://doi.org/10.3390/metabo9010003
Oberacher H, Reinstadler V, Kreidl M, Stravs MA, Hollender J, Schymanski EL. Annotating Nontargeted LC-HRMS/MS Data with Two Complementary Tandem Mass Spectral Libraries. Metabolites. 2019; 9(1):3. https://doi.org/10.3390/metabo9010003
Chicago/Turabian StyleOberacher, Herbert, Vera Reinstadler, Marco Kreidl, Michael A. Stravs, Juliane Hollender, and Emma L. Schymanski. 2019. "Annotating Nontargeted LC-HRMS/MS Data with Two Complementary Tandem Mass Spectral Libraries" Metabolites 9, no. 1: 3. https://doi.org/10.3390/metabo9010003
APA StyleOberacher, H., Reinstadler, V., Kreidl, M., Stravs, M. A., Hollender, J., & Schymanski, E. L. (2019). Annotating Nontargeted LC-HRMS/MS Data with Two Complementary Tandem Mass Spectral Libraries. Metabolites, 9(1), 3. https://doi.org/10.3390/metabo9010003