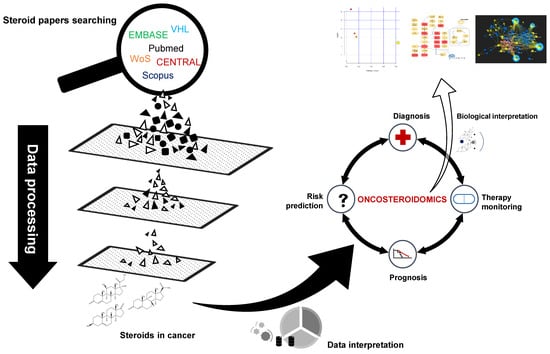
Steroidomics for the Prevention, Assessment, and Management of Cancers: A Systematic Review and Functional Analysis
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Literature and Search Strategies
2.2. Characteristics of the Included Studies
2.3. Sample Preparation and Analytical Procedures of the Included Studies
2.4. Steroids and the Prevention, Assessment, and Management of Cancer Patients
2.5. Assessment of Reporting Methodology Quality
2.6. Steroid Profiling Pathway Analysis and Network Analysis
3. Discussion
4. Materials and Methods
4.1. Systematic Literature Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Quality Assessment of Included Studies
4.5. Steroid Functional Analysis and Pathway Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic Phenotyping in Health and Disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beger, R.D. A review of applications of metabolomics in cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Mo, C.; Lee, J.H.; Long, N.P.; Dong, Z.; Li, J.; Lim, J.; Kwon, S.W. The integration of multi-platform MS-based metabolomics and multivariate analysis for the geographical origin discrimination of Oryza sativa L. J. Food Drug Anal. 2018, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kotłowska, A. Application of Steroid Hormone Metabolomics in Search of Biomarkers in Clinical Research. Drug Dev. Res. 2012, 73, 381–389. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, D.-H.; Long, N.P.; Luu, T.-T.-T.; Anh, N.H.; Kwon, S.W.; Lee, S.K. The Dominant Role of Forkhead Box Proteins in Cancer. Int. J. Mol. Sci. 2018, 19, 3279. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Yoon, S.J.; Long, N.P.; Jung, K.-H.; Kim, H.M.; Hong, Y.J.; Fang, Z.; Kim, S.J.; Kim, T.J.; Anh, N.H.; Hong, S.-S.; et al. Systemic and Local Metabolic Alterations in Sleep-Deprivation-Induced Stress: A Multiplatform Mass-Spectrometry-Based Lipidomics and Metabolomics Approach. J. Proteome Res. 2019. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.M.R.; Diaz-Rubio, M.E.; Chiou, A.E.; Loh, H.-C.; Zhang, S.; Masic, A.; Fischbach, C.; Estroff, L.A. Mapping and Profiling Lipid Distribution in a 3D Model of Breast Cancer Progression. ACS Cent. Sci. 2019, 5, 768–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.P.; Lee, W.J.; Hong, J.Y.; Lee, S.B.; Park, J.H.; Kim, D.; Park, S.; Park, C.-S.; Park, S.-W.; Kwon, S.W. Novel Approach for Analysis of Bronchoalveolar Lavage Fluid (BALF) Using HPLC-QTOF-MS-Based Lipidomics: Lipid Levels in Asthmatics and Corticosteroid-Treated Asthmatic Patients. J. Proteome Res. 2014, 13, 3919–3929. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Long, N.P.; Jung, J.; Kim, T.J.; Na, E.; Kang, Y.P.; Kwon, S.W.; Jang, J. Integrative lipidomic and transcriptomic analysis of X-linked adrenoleukodystrophy reveals distinct lipidome signatures between adrenomyeloneuropathy and childhood cerebral adrenoleukodystrophy. Biochem. Biophys. Res. Commun. 2019, 508, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hechter, O.; Halkerston, I.D.K. Effects of Steroid Hormones on Gene Regulation and Cell Metabolism. Annu. Rev. Physiol. 1965, 27, 133–162. [Google Scholar] [CrossRef] [PubMed]
- Jeanneret, F.; Tonoli, D.; Rossier, M.F.; Saugy, M.; Boccard, J.; Rudaz, S. Evaluation of steroidomics by liquid chromatography hyphenated to mass spectrometry as a powerful analytical strategy for measuring human steroid perturbations. J. Chromatogr. A 2016, 1430, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Alferez, D.G.; Simões, B.M.; Howell, S.J.; Clarke, R.B. The Role of Steroid Hormones in Breast and Effects on Cancer Stem Cells. Curr. Stem Cell Rep. 2018, 4, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Lorente, D.; Omlin, A.; Ferraldeschi, R.; Pezaro, C.; Perez, R.; Mateo, J.; Altavilla, A.; Zafeirou, Z.; Tunariu, N.; Parker, C.; et al. Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone. Br. J. Cancer 2014, 111, 2248–2253. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.; Kerstens, M.N.; Kema, I.P.; Willems, T.P.; Haak, H.R. Diagnostic Value of Urinary Steroid Profiling in the Evaluation of Adrenal Tumors. Horm Cancer 2015, 6, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Schrepf, A.; Thaker, P.H.; Goodheart, M.J.; Bender, D.; Slavich, G.M.; Dahmoush, L.; Penedo, F.; DeGeest, K.; Mendez, L.; Lubaroff, D.M.; et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology 2015, 53, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungenast, F.; Thalhammer, T. Estrogen Biosynthesis and Action in Ovarian Cancer. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, L.; Baczek, T.; Belka, M.; Fel, A.; Markuszewski, M.; Struck, W.; Markuszewski, M.; Kaliszan, R. Steroid profiles as potential biomarkers in patients with urogenital tract cancer for diagnostic investigations analyzed by liquid chromatography coupled to mass spectrometry. J. Pharm. Biomed. Anal. 2013, 73, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, C.; Pozo, O.J.; Marcos, J. GC/MS in Recent Years Has Defined the Normal and Clinically Disordered Steroidome: Will It Soon Be Surpassed by LC/Tandem MS in This Role? J. Endocr. Soc. 2018, 2, 974–996. [Google Scholar] [CrossRef] [PubMed]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, S.; Kunz, M.; Kurlbaum, M.; Vey, J.; Kendl, S.; Deutschbein, T.; Hahner, S.; Fassnacht, M.; Dandekar, T.; Kroiss, M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019, 180, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, J.M.; Bancos, I.; Bancos, C.; Singh, R.D.; Avula, A.V.; Young, W.F.; Grebe, S.K.; Singh, R.J. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin. Chem. 2017, 63, 1824–1835. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.R.; Ghataore, L.; Couchman, L.; Vincent, R.P.; Whitelaw, B.; Lewis, D.; Diaz-Cano, S.; Galata, G.; Schulte, K.M.; Aylwin, S.; et al. A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma. Clin. Chem. 2017, 63, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Zhan, Q.; Lv, L.; Zhang, H.; Hong, Z.; Li, Y.; Xu, H.; Chai, Y.; Zhao, L.; Zhang, G. Steroid hormone profiles plus α-fetoprotein for diagnosing primary liver cancer by liquid chromatography tandem mass spectrometry. Clinica Chimica Acta 2016, 457, 92–98. [Google Scholar] [CrossRef]
- Velikanova, L.I.; Shafigullina, Z.R.; Lisitsin, A.A.; Vorokhobina, N.V.; Grigoryan, K.; Kukhianidze, E.A.; Strelnikova, E.G.; Krivokhizhina, N.S.; Krasnov, L.M.; Fedorov, E.A.; et al. Different Types of Urinary Steroid Profiling Obtained by High-Performance Liquid Chromatography and Gas Chromatography-Mass Spectrometry in Patients with Adrenocortical Carcinoma. Horm Cancer 2016, 7, 327–335. [Google Scholar] [CrossRef]
- Dai, W.; Yin, P.; Chen, P.; Kong, H.; Luo, P.; Xu, Z.; Lu, X.; Xu, G. Study of urinary steroid hormone disorders: Difference between hepatocellular carcinoma in early stage and cirrhosis. Anal. Bioanal. Chem. 2014, 406, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Perna, V.; Taylor, N.F.; Dworakowska, D.; Schulte, K.M.; Aylwin, S.; Al-Hashimi, F.; Diaz-Cano, S.J. Adrenocortical adenomas with regression and myelolipomatous changes: Urinary steroid profiling supports a distinctive benign neoplasm. Clin. Endocrinol. 2014, 81, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, L.; Belka, M.; Baczek, T.; Ruszkowski, M.; Struck, W.; Markuszewski, M.; Kaliszan, R.; Markuszewski, M. Advanced assessment of the endogenous hormone level as a potential biomarker of the urogenital tract cancer. Comb. Chem. High Throughput Screen. 2013, 16, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libe, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Bufa, A.; Biro, I.; Poor, V.; Molnar, G.; Kovacs, K.A.; Felinger, A.; Jeges, S.; Kilar, F.; Gocze, P.M. Altered urinary profiles of endogenous steroids in postmenopausal women with adenocarcinoma endometrii. Gynecol. Endocrinol. 2010, 26, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bufa, A.; Poór, V.; Bálint, A.; Molnár, S.; Jeges, S.; Pótó, L.; Gőcze, P.; Kilár, F. Endogenous Urinary Steroids in Postmenopausal Women with Epithelial Ovarian Cancer. Chromatographia 2008, 68, 131–135. [Google Scholar] [CrossRef]
- Drafta, D.; Proca, E.; Zamfir, V.; Schindler, A.E.; Neacsu, E.; Stroe, E. Plasma steroids in benign prostatic hypertrophy and carcinoma of the prostate. J. Steroid Biochem. 1982, 17, 689–693. [Google Scholar] [CrossRef]
- Trabert, B.; Michels, K.A.; Anderson, G.L.; Brinton, L.A.; Falk, R.T.; Geczik, A.M.; Harris, H.R.; Pan, K.; Pfeiffer, R.M.; Qi, L.; et al. Circulating androgens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol. Prev. Biomark. 2019, 25, 648–656. [Google Scholar] [CrossRef]
- Petrick, J.L.; Falk, R.T.; Hyland, P.L.; Caron, P.; Pfeiffer, R.M.; Wood, S.N.; Dawsey, S.M.; Abnet, C.C.; Taylor, P.R.; Guillemette, C.; et al. Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study. PLoS One 2018, 13, e0190325. [Google Scholar] [CrossRef]
- Petrick, J.L.; Hyland, P.L.; Caron, P.; Falk, R.T.; Pfeiffer, R.M.; Dawsey, S.M.; Abnet, C.C.; Taylor, P.R.; Weinstein, S.J.; Albanes, D.; et al. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Esophageal/Gastric Cardia Adenocarcinoma Among Men. J. Natl. Cancer Inst. 2019, 111, 34–41. [Google Scholar] [CrossRef]
- Sampson, J.N.; Falk, R.T.; Schairer, C.; Moore, S.C.; Fuhrman, B.J.; Dallal, C.M.; Bauer, D.C.; Dorgan, J.F.; Shu, X.O.; Zheng, W.; et al. Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res. 2017, 77, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Trabert, B.; Anderson, G.L.; Falk, R.T.; Felix, A.S.; Fuhrman, B.J.; Gass, M.L.; Kuller, L.H.; Pfeiffer, R.M.; Rohan, T.E.; et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol. Prev. Biomark. 2016, 25, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.C.; Matthews, C.E.; Ou Shu, X.; Yu, K.; Gail, M.H.; Xu, X.; Ji, B.T.; Chow, W.H.; Cai, Q.; Li, H.; et al. Endogenous Estrogens, Estrogen Metabolites, and Breast Cancer Risk in Postmenopausal Chinese Women. J. Natl Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabert, B.; Brinton, L.A.; Anderson, G.L.; Pfeiffer, R.M.; Falk, R.T.; Strickler, H.D.; Sliesoraitis, S.; Kuller, L.H.; Gass, M.L.; Fuhrman, B.J.; et al. Circulating Estrogens and Postmenopausal Ovarian Cancer Risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol. Prev. Biomark. 2016, 25, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Dallal, C.M.; Lacey, J.V., Jr.; Pfeiffer, R.M.; Bauer, D.C.; Falk, R.T.; Buist, D.S.; Cauley, J.A.; Hue, T.F.; LaCroix, A.Z.; Tice, J.A.; et al. Estrogen Metabolism and Risk of Postmenopausal Endometrial and Ovarian Cancer: The B approximately FIT Cohort. Horm Cancer 2016, 7, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Schairer, C.; Fuhrman, B.J.; Boyd-Morin, J.; Genkinger, J.M.; Gail, M.H.; Hoover, R.N.; Ziegler, R.G. Quantifying the Role of Circulating Unconjugated Estradiol in Mediating the Body Mass Index-Breast Cancer Association. Cancer Epidemiol. Prev. Biomark. 2016, 25, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; Pinsky, P.F.; Grubb, R.L.; Falk, R.T.; Hsing, A.W.; Chu, L.; Meyer, T.; Veenstra, T.D.; Xu, X.; Yu, K.; et al. Sex steroid hormone metabolism in relation to risk of aggressive prostate cancer. Cancer Epidemiol. Prev. Biomark. 2014, 23, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.T.; Brinton, L.A.; Dorgan, J.F.; Fuhrman, B.J.; Veenstra, T.D.; Xu, X.; Gierach, G.L. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: A nested case-control study. Breast Cancer Res. 2013, 15, R34. [Google Scholar] [CrossRef] [PubMed]
- Dallal, C.M.; Tice, J.A.; Buist, D.S.M.; Bauer, D.C.; Lacey, J.V., Jr.; Cauley, J.A.; Hue, T.F.; Lacroix, A.; Falk, R.T.; Pfeiffer, R.M.; et al. Estrogen metabolism and breast cancer risk among postmenopausal women: A case-cohort study within B~FIT. Carcinogenesis 2014, 35, 346–355. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Schairer, C.; Gail, M.H.; Boyd-Morin, J.; Xu, X.; Sue, L.Y.; Buys, S.S.; Isaacs, C.; Keefer, L.K.; Veenstra, T.D.; et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 2012, 104, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Audet-Walsh, E.; Lepine, J.; Gregoire, J.; Plante, M.; Caron, P.; Tetu, B.; Ayotte, P.; Brisson, J.; Villeneuve, L.; Belanger, A.; et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: Association with risk and relationship to clinical characteristics. J. Clin. Endocrinol. Metab. 2011, 96, E330–E339. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gaikwad, N.W.; Meza, J.; Cavalieri, E.L.; Muti, P.; Trock, B.; Rogan, E.G. Novel biomarkers for risk of prostate cancer: Results from a case-control study. Prostate 2009, 69, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Levesque, E.; Caron, P.; Lacombe, L.; Turcotte, V.; Simonyan, D.; Fradet, Y.; Aprikian, A.; Saad, F.; Carmel, M.; Chevalier, S.; et al. A Comprehensive Analysis of Steroid Hormones and Progression of Localized High-Risk Prostate Cancer. Cancer Epidemiol. Prev. Biomark. 2019. [Google Scholar] [CrossRef] [PubMed]
- Audet-Delage, Y.; Gregoire, J.; Caron, P.; Turcotte, V.; Plante, M.; Ayotte, P.; Simonyan, D.; Villeneuve, L.; Guillemette, C. Estradiol metabolites as biomarkers of endometrial cancer prognosis after surgery. J. Steroid Biochem. Mol. Biol. 2018, 178, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plenis, A.; Miękus, N.; Olędzka, I.; Bączek, T.; Lewczuk, A.; Woźniak, Z.; Koszałka, P.; Seroczyńska, B.; Skokowski, J. Chemometric evaluation of urinary steroid hormone levels as potential biomarkers of neuroendocrine tumors. Molecules 2013, 18, 12857–12876. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, É.; Laverdière, I.; Lacombe, L.; Caron, P.; Rouleau, M.; Turcotte, V.; Têtu, B.; Fradet, Y.; Guillemette, C. Importance of 5α-Reductase Gene Polymorphisms on Circulating and Intraprostatic Androgens in Prostate Cancer. Clin. Cancer Res. 2014, 20, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.S.; Bulbrook, R.D.; Hayward, J.L.; Millis, R.R. Urinary androgen metabolites and recurrence rates in early breast cancer. Eur. J. Cancer Clin. Oncol. 1982, 18, 447–451. [Google Scholar] [CrossRef]
- Zang, X.; Jones, C.M.; Long, T.Q.; Monge, M.E.; Zhou, M.; Walker, L.D.; Mezencev, R.; Gray, A.; McDonald, J.F.; Fernandez, F.M. Feasibility of detecting prostate cancer by ultraperformance liquid chromatography-mass spectrometry serum metabolomics. J. Proteome Res. 2014, 13, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Peng, J.-S.; Dong-Sheng, Y.; Yang, Z.-L.; Liu, H.-L.; Zeng, Y.-K.; Shi, X.-P.; Lu, B.-Y. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Braz. J. Med. Biol. Res. 2011, 45, 78–85. [Google Scholar] [CrossRef]
- Moore, S.C.; Playdon, M.C.; Sampson, J.N.; Hoover, R.N.; Trabert, B.; Matthews, C.E.; Ziegler, R.G. A Metabolomics Analysis of Body Mass Index and Postmenopausal Breast Cancer Risk. J. Natl. Cancer Inst. 2018, 110, 588–597. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Prospective serum metabolomic profile of prostate cancer by size and extent of primary tumor. Oncotarget 2017, 8, 45190–45199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The α-tocolpherol, β-carotene cancer prevention (ATBC) study. Int. J. Cancer 2015, 137, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Moore, S.C.; Derkach, A.; Hua, X.; Mondul, A.M.; Sampson, J.N.; Albanes, D. Pre-diagnostic Serum Metabolomic Profiling of Prostate Cancer Survival. J. Gerontol. Ser. A 2018. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Liu, Y.; Yin, P.; Zeng, Z.; Huang, Q.; Kong, H.; Lu, X.; Zhong, L.; Zhang, Z.; Xu, G. Study of induction chemotherapy efficacy in oral squamous cell carcinoma using pseudotargeted metabolomics. J. Proteome Res. 2014, 13, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, Y.; Yin, P.; Zeng, Z.; Li, J.; Lu, X.; Zheng, L.; Xu, G. Metabolic profiling study of early and late recurrence of hepatocellular carcinoma based on liquid chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 966, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.H. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ghataore, L.; Chakraborti, I.; Aylwin, S.J.; Schulte, K.M.; Dworakowska, D.; Coskeran, P.; Taylor, N.F. Effects of mitotane treatment on human steroid metabolism: Implications for patient management. Endocr. Connect. 2012, 1, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Saylor, P.J.; Karoly, E.D.; Smith, M.R. Prospective study of changes in the metabolomic profiles of men during their first three months of androgen deprivation therapy for prostate cancer. Clin. Cancer Res. 2012, 18, 3677–3685. [Google Scholar] [CrossRef]
- Kaushik, A.K.; DeBerardinis, R.J. Applications of metabolomics to study cancer metabolism. Biochimica Biophysica Acta BBA Rev. Cancer 2018, 1870, 2–14. [Google Scholar] [CrossRef]
- Long, N.P.; Yoon, S.J.; Anh, N.H.; Nghi, T.D.; Lim, D.K.; Hong, Y.J.; Hong, S.-S.; Kwon, S.W.J.M. A systematic review on metabolomics-based diagnostic biomarker discovery and validation in pancreatic cancer. Metabolomics 2018, 14, 109. [Google Scholar] [CrossRef]
- Erben, V.; Bhardwaj, M.; Schrotz-King, P.; Brenner, H. Metabolomics Biomarkers for Detection of Colorectal Neoplasms: A Systematic Review. Cancers 2018, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: A systematic review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Peitzsch, M.; Kaden, D.; Langton, K.; Pamporaki, C.; Masjkur, J.; Tsatsaronis, G.; Mangelis, A.; Williams, T.A.; Reincke, M.; et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clinica Chimica Acta 2017, 470, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.W.; Botelho, J.C.; Shacklady, C.; Smith, A.; Myers, G.L. CDC project on standardizing steroid hormone measurements. Steroids 2008, 73, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Ceglarek, U.; Werner, M.; Kortz, L.; Körner, A.; Kiess, W.; Thiery, J.; Kratzsch, J. Preclinical challenges in steroid analysis of human samples. J. Steroid Biochem. Mol. Biol. 2010, 121, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, O.; Gagnon, J.; Rankinen, T.; Thompson, P.A.; Hong, Y.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Age, body mass index, race and other determinants of steroid hormone variability: The HERITAGE Family Study. Eur. J. Endocrinol. 2001, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.G.; Altman, D.G.; De Stavola, B.L. Quantification of the completeness of follow-up. Lancet 2002, 359, 1309–1310. [Google Scholar] [CrossRef]
- Von Allmen, R.S.; Weiss, S.; Tevaearai, H.T.; Kuemmerli, C.; Tinner, C.; Carrel, T.P.; Schmidli, J.; Dick, F. Completeness of Follow-Up Determines Validity of Study Findings: Results of a Prospective Repeated Measures Cohort Study. PLoS ONE 2015, 10, e0140817. [Google Scholar] [CrossRef]
- Srinivas, P.R.; Kramer, B.S.; Srivastava, S. Trends in biomarker research for cancer detection. Lancet Oncol. 2001, 2, 698–704. [Google Scholar] [CrossRef]
- Zhao, M.; Baker, S.D.; Yan, X.; Zhao, Y.; Wright, W.W.; Zirkin, B.R.; Jarow, J.P. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids 2004, 69, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kotlowska, A.; Szefer, P. Recent Advances and Challenges in Steroid Metabolomics for Biomarker Discovery. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.G.; Matthew, S.; Auchus, R.J. Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases. Horm. Cancer 2011, 2, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Tavita, N.; Greaves, R.F. Systematic review of serum steroid reference intervals developed using mass spectrometry. Clin. Biochem. 2017, 50, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N.; Völzke, H.; Rosskopf, D.; Steveling, A.; Krebs, A.; Nauck, M.; Wallaschofski, H. Reference Ranges for Serum Dehydroepiandrosterone Sulfate and Testosterone in Adult Men. J. Androl. 2008, 29, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartmann, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Long, N.P.; Park, S.; Anh, N.H.; Nghi, T.D.; Yoon, S.J.; Park, J.H.; Lim, J.; Kwon, S.W. High-Throughput Omics and Statistical Learning Integration for the Discovery and Validation of Novel Diagnostic Signatures in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 296. [Google Scholar] [CrossRef]
- Long, N.P.; Jung, K.H.; Yoon, S.J.; Anh, N.H.; Nghi, T.D.; Kang, Y.P.; Yan, H.H.; Min, J.E.; Hong, S.S.; Kwon, S.W. Systematic assessment of cervical cancer initiation and progression uncovers genetic panels for deep learning-based early diagnosis and proposes novel diagnostic and prognostic biomarkers. Oncotarget 2017, 8, 109436–109456. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Hu, C.; Zhang, C.; Kovatcheva-Datchary, P.; Yu, D.; Liu, S.; Ren, F.; Wang, X.; Li, Y.; et al. Integrated Metabolomics and Lipidomics Analyses Reveal Metabolic Reprogramming in Human Glioma with IDH1 Mutation. J. Proteome Res. 2018. [Google Scholar] [CrossRef]
- Zhu, R.; Zhao, Q.; Zhao, H.; Ma, S. Integrating multidimensional omics data for cancer outcome. Biostatistics 2016, 17, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Shi, X.; Zhao, Q.; Krauthammer, M.; Rothberg, B.E.; Ma, S. Integrated analysis of multidimensional omics data on cutaneous melanoma prognosis. Genomics 2016, 107, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, J.; Claggett, B.L.; Henglin, M.; Kim, A.; Ovsak, G.; Kim, N.; Deng, K.; Rao, K.; Tyagi, O.; Watrous, J.D.; et al. Statistical Workflow for Feature Selection in Human Metabolomics Data. Metabolites 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Kamrath, C.; Wudy, S.A.; Krone, N. Steroid biochemistry. Endocrine Dev. 2014, 27, 41–52. [Google Scholar] [CrossRef]
- Dai, W.; Huang, Q.; Yin, P.; Li, J.; Zhou, J.; Kong, H.; Zhao, C.; Lu, X.; Xu, G. Comprehensive and Highly Sensitive Urinary Steroid Hormone Profiling Method Based on Stable Isotope-Labeling Liquid Chromatography–Mass Spectrometry. Anal. Chem. 2012, 84, 10245–10251. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Yazdani, A.; Karniadakis, G.E.; Mantzoros, C. Omics, big data and machine learning as tools to propel understanding of biological mechanisms and to discover novel diagnostics and therapeutics. Metab. Clin. Exp. 2018, 87, A1–A9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.H.; Huang, M.; Kelly, R.S.; Benedetti, E.; Siddiqui, J.K.; Zeleznik, O.A.; Pereira, A.; Herrington, D.; Wheelock, C.E.; Krumsiek, J.; et al. Integration of Metabolomic and Other Omics Data in Population-Based Study Designs: An Epidemiological Perspective. Metabolites 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.Z., Jr.; Kuhn, E.; Morrison, J.C.; Bahadirli-Talbott, A.; Smith-Sehdev, A.; Kurman, R.J. Steroid hormone synthesis by the ovarian stroma surrounding epithelial ovarian tumors: A potential mechanism in ovarian tumorigenesis. Mod. Pathol. 2017, 30, 563. [Google Scholar] [CrossRef]
- Cirillo, N.; Morgan, D.J.; Pedicillo, M.C.; Celentano, A.; Lo Muzio, L.; McCullough, M.J.; Prime, S.S. Characterisation of the cancer-associated glucocorticoid system: Key role of 11β-hydroxysteroid dehydrogenase type 2. Br. J. Cancer 2017, 117, 984. [Google Scholar] [CrossRef]
- Klinge, C.M.; Clark, B.J.; Prough, R.A. Chapter One—Dehydroepiandrosterone Research: Past, Current, and Future. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Lopez-Marure, R.; Zapata-Gomez, E.; Rocha-Zavaleta, L.; Aguilar, M.C.; Espinosa Castilla, M.; Melendez Zajgla, J.; Meraz-Cruz, N.; Huesca-Gomez, C.; Gamboa-Avila, R.; Gomez-Gonzalez, E.O. Dehydroepiandrosterone inhibits events related with the metastatic process in breast tumor cell lines. Cancer Biol. Ther. 2016, 17, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Calderón, Y.N.; López-Marure, R. Dehydroepiandrosterone Inhibits Proliferation and Suppresses Migration of Human Cervical Cancer Cell Lines. Anticancer Res. 2014, 34, 4039–4044. [Google Scholar] [PubMed]
- Fournier, A.; Roddam, A.; Olsen, A.; Thiebaut, A.; Tjønneland, A.; Trichopoulou, A.; Gurrea, A.B.; Biessy, C.; van Gils, C.H.; Gonzalez, C.A.; et al. Serum Sex Steroids in Premenopausal Women and Breast Cancer Risk Within the European Prospective Investigation into Cancer and Nutrition (EPIC). JNCI J. Natl Cancer Inst. 2005, 97, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P.J.; Simons, C.; Lain, R.L. Inhibitors of steroidogenesis as agents for the treatment of hormone-dependent cancers. Expert Opin. Ther. Pat. 2001, 11, 789–824. [Google Scholar] [CrossRef]
- Fleseriu, M.; Castinetti, F. Updates on the role of adrenal steroidogenesis inhibitors in Cushing’s syndrome: A focus on novel therapies. Pituitary 2016, 19, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.; Njar, V.; Macedo, L.F.; Vasaitis, T.S.; Sabnis, G. The Coffey Lecture: Steroidogenic enzyme inhibitors and hormone dependent cancer. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, S.; Pham, S.; Ming, D.-S.; Chin, M.Y.; Adomat, H.; Hurtado-Coll, A.; Gleave, M.E.; Guns, E.S.T. Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models. Cancers 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Fromont, G.; Yacoub, M.; Valeri, A.; Mangin, P.; Vallancien, G.; Cancel-Tassin, G.; Cussenot, O. Differential expression of genes related to androgen and estrogen metabolism in hereditary versus sporadic prostate cancer. Cancer Epidemiol. Prev. Biomark. 2008, 17, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takayama, K.; Takahashi, S.; Inoue, S. Estrogen and Androgen Blockade for Advanced Prostate Cancer in the Era of Precision Medicine. Cancers 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Yao, S.; Till, C.; Kristal, A.R.; Goodman, P.J.; Hsing, A.W.; Tangen, C.M.; Platz, E.A.; Stanczyk, F.Z.; Reichardt, J.K.V.; et al. Effect of finasteride on serum androstenedione and risk of prostate cancer within the prostate cancer prevention trial: Differential effect on high- and low-grade disease. Urology 2015, 85, 616–620. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras, B.; Porta, M.; Marquez, S.; Pollan, M.; Parker, L.A.; Hernandez-Aguado, I. QUADOMICS: An adaptation of the Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of ’-omics’-based technologies. Clin. Biochem. 2008, 41, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Caraus, I.; Xia, J.; Li, C.; Wishart, D.S.; Bourque, G.; Li, S. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xia, J. OmicsNet: A web-based tool for creation and visual analysis of biological networks in 3D space. Nucleic Acids Res. 2018, 46, W514–W522. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2010, 38, D480–D487. [Google Scholar] [CrossRef]



| Study and Year of Publication | Sample Collection | Cohort Allocation | Aim | Patients | Controls | Follow-Up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Diagnosis | No. | Age | M/F | Stage | Hormone Treatment | Type | Match | No. | Age | M/F | |||||
| Schweitzer et al. (2018) [26] | Prospective | ENSAT | Diagnosis | ACC | Pathologically confirmed | 42 | M: 57; R: 20–80 | 15/27 | I-V | NA | ACA | Yes | 66 | M: 58; R: 21–81 | 29/37 | No |
| Hines et al. (2017) [27] | Prospective | US | Diagnosis | ACC | Pathologically confirmed | 5 | NA | NA | NA | No | H, ACA | No | 114, 61 | M1: 42, 47; R1: 24–83, 25–83 | 48/66 | NA |
| Taylor et al. (2017) [28] | Prospective | UK | Diagnosis | ACC | Pathologically confirmed | 10 | M: 59; R: 47–69 | 4/6 | NA | NA | ACA, PPC/PGL, NFAA | Yes | 7, 15, 16 | M: 68, 50, 62; R: 66–70, 44–66, 48–72 | 4/3; 8/7; 6/10 | NA |
| Qian et al. (2016) [29] | Prospective | China | Diagnosis | Primary LC | AJCC | 66 | m: 57.5; SD: 9.6 | 66/0 | I-II | No | CL, H | No | 59, 65 | m: 50.6, 53.6; SD: 12.5, 15.4 | 59/0; 65/0 | NA |
| Velikanova et al. (2016) [30] | Prospective | Russia | Diagnosis | ACC | Pathologically confirmed | 31 | M: 43; R: 33–57 | 8/23 | NA | Yes | ACA-HNA, ACA-CS, H | No | 52, 44, 25 | M: 55, 48; R: 50–61, 21–54 | 17/35; 18/26 | NA |
| Kerkhofs et al. (2015) [20] | Retrospective | Netherland | Diagnosis | ACC | Pathologically confirmed2 | 27 | m: 57; SD: 14 | 8/19 | II-IV | NA | ACA function, ACA non function | No | 22, 85 | m: 50, 58; SD: 12, 12 | 6/16; 28/57 | Yes |
| Dai et al. (2014) [31] | Prospective | China | Diagnosis | HCC | Pathologically confirmed | 28 | NA | NA | I3 | NA | H, CL | NA | 21, 21 | NA | NA | NA |
| Perna et al. (2014) [32] | Prospective | UK | Diagnosis | ACC | Pathologically confirmed | 13 | m: 51.7; SD: 16.2 | 4/9 | NA | NA | ACA-RML, ACA | No | 7, 11 | m: 70.14, 54.3; SD: 8.84, 12.35 | 4/3; 2/9 | NA |
| Konieczna et al. (2013) [33] | Prospective | Poland | Diagnosis | BlC, KC, PC, TC, others | Pathologically confirmed | 58, 11, 9, 3, 114 | m: >40 | 46/12; 7/4; NA; NA; NA | NA | NA | H | No | 100 | m: >40 | 61/39 | NA |
| Konieczna et al. (2013) [23] | Prospective | Poland | Diagnosis | BlC, KC, PC, TC, others | NA | 47, 10, 7, 3, 104 | m: 65.00; SD: 10.40 | 17/60 | NA | No | H | Yes | 77 | m: 46.97; SD: 18.51 | 38/39 | NA |
| Arlt et al. (2011) [34] | Retrospective | ENSAT | Diagnosis | ACC | Pathologically confirmed | 45 | M: 55; R: 20–80 | 24/21 | NA | No | ACA, H | NA | 102, 88 | M: 60; R: 19–84; 18–60 | 39/63; 26/62 | Yes |
| Bufa et al. (2010) [35] | Prospective | Hungary | Diagnosis | AE | NA | 13 | m: 67.9; SD: 8.5 | 0/13 | NA | NA | H | Yes | 10 | m: 58.7; SD: 6.2 | 0/10 | NA |
| Bufa et al. (2008) [36] | Prospective | Hungary | Diagnosis | EOC | NA | 15 | m: 60.4; SD: 5.1 | 0/15 | NA | NA | H | Yes | 10 | m: 58.7; SD: 6.2 | 0/10 | NA |
| Drafta et al. (1982) [37] | Prospective | Romania | Diagnosis | PC | UICC 1974 and VACRG | 32 | m: 67; R: 51–79 | 32/0 | I-IV | NA | BPH, H | Yes5 | 54, 63 | m: 68, 66; R: 50–78, 50–79 | 54/0; 63/0 | NA |
| Trabert et al. (2019) [38] | Retrospective | WHI-OS | Risk prediction | OC | NA | 169 | m: 64.1; SD: 7.2 | 0/169 | NA | No | H | Yes | 410 | m: 64.3; SD: 7.2 | 0/410 | Yes |
| Petrick et al. (2018) [39] | Retrospective | Northern Ireland, Ireland | Risk prediction | EA | Pathologically confirmed | 172 | m: 64.3; SD: 10.9 | 172/0 | NA | No | H | Yes | 185 | m: 63.5; SD: 12.6 | 185/0 | NA |
| Petrick et al. (2018) [40] | Retrospective | PLCO, ATBC, CPS-II nutrition cohort | Risk prediction | EA/GCA | NA | 259 | m: 62.0; SD: 6.6 | 259/0 | NA | No | H | Yes | 259 | m: 61.0; SD: 6.6 | 259/0 | NA |
| Sampson et al. (2017) [41] | Retrospective | PLCO, US, B-FIT, SWHS | Risk prediction | BC | NA | 1298 | NA | 0/1298 | NA | No | H | Yes | 1524 | NA | 0/1524 | Yes |
| Brinton et al. (2016) [42] | Retrospective | WHI-OS | Risk prediction | EC | NA | 313 | m: 64.5; SD: 7.0 | 0/313 | NA | No | H | Yes | 354 | m: 64.0; SD: 7.0 | 0/354 | Yes |
| Moore et al. (2016) [43] | Retrospective | China | Risk prediction | BC | NA | 399 | NA | 0/399 | NA | No | H | Yes | 399 | NA | 0/399 | Yes |
| Trabert et al. (2016) [44] | Retrospective | WHI-OS | Risk prediction | OC | NA | 169 | m: 64.1; SD: 7.2 | 0/169 | NA | No | H | Yes | 412 | m: 64.3; SD: 7.2 | 0/412 | Yes |
| Dallal et al. (2016) [45] | Retrospective | B-FIT | Risk prediction | EC | NA | 66 | m: 67.5; SD: 5.6 | 0/66 | NA | No | H | No | 346 | m: 67.0; SD: 6.2 | 0/346 | Yes |
| - | Retrospective | B-FIT | Risk prediction | OC | NA | 67 | m: 68.5; SD: 5.7 | 0/67 | NA | No | H | No | 416 | m: 67.0; SD: 6.3 | 0/416 | Yes |
| Schairer et al. (2015) [46] | Retrospective | PLCO | Risk prediction | BC (estrogen receptor positive) | NA | 193 | R: 55–74 | 0/193 | NA | No | H | Yes | 268 | NA | 0/268 | Yes |
| Black et al. (2014) [47] | Retrospective | PLCO | Risk prediction | PC | NA | 195 | R: 55–70 | 195/0 | III-IV | No | H | Yes | 195 | R: 55–70 | 195/0 | Yes |
| Falk et al. (2013) [48] | Retrospective | US | Risk prediction | BC | NA | 215 | NA | 0/215 | NA | No | H | Yes | 215 | NA | 0/215 | Yes |
| Dallal et al. (2013) [49] | Retrospective | B-FIT | Risk prediction | BC | NA | 407 | m: 67.2; SD: 5.7 | 0/407 | NA | No | H | No | 496 | m: 67.3; SD: 6.2 | 0/496 | Yes |
| Fuhrman et al. (2012) [50] | Retrospective | PLCO | Risk prediction | BC | NA | 277 | R: 55–74 | 0/277 | NA | No | H | No | 423 | R: 55–74 | 0/423 | Yes |
| Audet-Walsh et al. (2010) [51] | Retrospective | Canada | Risk prediction | EC | NA | 126 | m: 64.8; SD: 9.1 | 0/126 | I-IV | No | H | No | 110 | m: 58.3; SD: 5.6 | 0/110 | NA |
| Yang et al. (2009) [52] | Prospective | US | Risk prediction | PC | NA | 14 | m: 63.6; R: 50–83 | 14/0 | NA | NA | H | No | 125 | m: 64.8; R: 45–78 | 125/0 | NA |
| Lévesque et al. (2019) [53] | Retrospective | Canada | Prognosis | PC | NA | 17766 | m: 62.7; SD: 6.4 | 1776/0 | I-IV | No | PC | Yes | 17766 | m: 62.7; SD: 6.4 | 1776/0 | Yes |
| Audet-Delage et al. (2018) [54] | Prospective | Canada | Prognosis | EC | Pathologically confirmed | 246 | m: 65.1; SD: 8.9 | 0/246 | I-IV | No | EC7, H | Yes | 246, 110 | m: 65.1, 58.3; SD: 8.9, 5.6 | 0/246; 0/110 | Yes |
| Plenis et al. (2013) [55] | Prospective | Poland | Prognosis | NET | NA | 198 | m: 54.6; SD: 11.8 | 10/9 | NA | NA | H | Yes | 20 | m: 47.3; SD: 12.5 | 10/10 | NA |
| Lévesque et al. (2013) [56] | Prospective | Canada | Prognosis | PC | Pathologically confirmed | 5269 | m: 63.3; SD: 6.8 | NA | NA | No | NA | NA | NA | NA | NA | NA |
| Thomas et al. (1982) [57] | Prospective | UK | Prognosis | BC10 | Pathologically confirmed | 109 | NA | 0/109 | I-II | NA | BC11 | NA | 109 | NA | 0/109 | Yes |
| Zang el at. (2014) [58] | Prospective | US | Diagnosis | PC | NA | 64 | m: 59; R: 49–65 | 64/0 | NA | No | H | Yes | 50 | m: 50; R: 45–76 | 50/0 | NA |
| Song et al. (2012) [59] | Prospective | China | Diagnosis | GC | Pathologically confirmed | 30 | M: 63; R: 39-88 | 15/15 | I-IV | No | H | Yes | 30 | M: 62; R: 42–82 | 15/15 | No |
| Moore et al. (2018) [60] | Retrospective | PLCO | Risk prediction | BC | NA | 621 | R: 55–74 | 0/621 | NA | No | H | Yes | 621 | R: 55–74 | 0/621 | Yes |
| Huang et al. (2017) [61] | Retrospective | Finland | Risk prediction | PC | NA | 137 | m: 59.8, 58, 60.9 | 137/0 | II-IV | NA | H | Yes | 200 | m: 59.3 | 200/0 | NA |
| Mondul et al. (2015) [62] | Retrospective | ATBC | Risk prediction | PC | AJCC | 200 | m: 59.4 | 200/0 | III-IV | No | H | Yes | 200 | m: 59.3 | 200/0 | Yes |
| Huang et al. (2018) [63] | Retrospective | Finland | Prognosis | 3rd tertile of PC | AJCC | 1976, 12 | m: 69; R: 55–86 | 197/0 | I-IV | NA | 1st and 2nd tertile of PC | No | 1976, 12 | m: 69; R: 55–86 | 197/0 | Yes |
| Ye et al. (2014) [64] | Prospective | China | Prognosis | OSCC (S) | UICC 2002 | 11 | M: 52; R: 35–74 | 7/4 | III-IVA | No | OSCC (NS) | Yes | 21 | M: 53; R: 45–71 | 15/6 | NA |
| Zhou et al. (2014) [65] | Prospective | China | Prognosis | HCC | 6th TNM | 2213 | m: 47; SD: 12 | 19/3 | I-IIIB14 | NA | HCC | Yes | 18 | m: 45; SD: 11 | 15/3 | Yes |
| Miller et al. (2015) [66] | Prospective | US | Therapy monitoring | BC after limonene intervention | Pathologically confirmed | 406 | M: 58.5; IQR: 18.5 | 0/40 | IS-T1 | NA | BC before limonene intervention | Yes | 406 | M: 58.5, IQR: 18.5 | 0/40 | NA |
| Ghataore et al. (2012) [67] | Prospective | France | Therapy monitoring | ACC | Pathologically confirmed | 17 | M15: 50/47; R15: 26–66/20–76 | 6/1116 | NA | Yes | H | No | 40 | M15: 31/29; R15: 22–49/20–59 | 20/20 | Yes |
| Saylor et al. (2012) [68] | Prospective | US | Therapy monitoring | PC after ADT | NA | 36 | NA | 36/0 | NA | No | PC before ADT | Yes | 36 | NA | 36/0 | Yes |
| Steroid Compound | Biomarker Function in Cancer | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC | PC | BC | BlC | EC | LC | KC | TC | NET | OC | E/GC | ||
| Estradiol | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | [26,29,37,39,40,41,42,43,44,45,46,48,49,50,54] | ||||
| Dehydroepiandrosterone | ↑ | ↑ | ↑↓ | ↑ | ↑ | ↓ | [26,27,30,32,36,39,40,51,53,54,56] | |||||
| Cortisol | ↑ | ↑ | ↑ | ↓ | → | [23,26,27,28,30,31,33,37,55,68] | ||||||
| Pregnanetriol | ↑ | ↑ | ↑ | ↑ | [20,27,30,34,35,36] | |||||||
| Testosterone | ↑ | ↓ | ↑ | ↓ | ↑ | → | ↓ | [23,29,33,37,39,51,54,55,56] | ||||
| Estrone | ↑ | ↑ | ↑↓ | ↑ | ↑ | ↓ | [29,37,39,41,42,43,44,48,51,54] | |||||
| 2-methoxyestrone | ↑ | ↑ | ↑ | ↑ | [41,42,43,44,47,49] | |||||||
| Pregnanediol | ↑ | ↑ | [20,27,30,32,34,36] | |||||||||
| Androsterone | ↑ | ↑ | ↓ | ↑↓ | ↓ | [20,35,39,54,56,57] | ||||||
| Dehydroepiandrosterone sulfate | ↑ | ↑ | ↑ | ↑ | [26,28,51,53,54,65,68] | |||||||
| 2-hydroxyestrone | ↑ | ↑ | ↑ | ↑ | [41,42,43,44,45,48] | |||||||
| Estriol | ↑ | ↑ | ↑ | [41,42,43,44,48,49,54] | ||||||||
| 16-epiestriol | ↑ | ↑ | ↑ | [41,42,43,44,48,49] | ||||||||
| 16α-hydroxyestrone | ↑ | ↑ | ↑ | [41,42,43,44,45] | ||||||||
| Etiocholanolone | ↑ | ↑ | ↑ | [20,27,30,34,35,57] | ||||||||
| Androstenedione | ↑ | ↑ | ↑ | [26,28,51,54,58] | ||||||||
| Dihydrotestosterone | ↑ | ↑ | ↑ | ↓ | [26,39,51,54,56] | |||||||
| 16-ketoestradiol | ↑ | ↑ | ↑ | [41,42,43,44,49] | ||||||||
| Tetrahydrodeoxycortisol | ↑ | [20,27,30,34] | ||||||||||
| Cortisone | ↑ | ↑ | → | [23,27,30,33,55] | ||||||||
| Progesterone | ↑ | ↓ | ↓ | ↓ | ↓ | → | [23,26,33,55] | |||||
| Androstenediol | ↑ | ↓ | [39,51,53,54] | |||||||||
| 2-hydroxyestrone-3-methyl ether | ↑ | ↑ | [41,42,43,48] | |||||||||
| 4-hydroxyestrone | ↑ | ↑ | ↑ | [41,42,43,44,45] | ||||||||
| 4-methoxyestrone | ↑ | ↑ | ↑ | [42,44,47,54] | ||||||||
| 17-epiestriol | ↑ | ↑ | [41,42,43,45,49] | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, N.H.; Long, N.P.; Kim, S.J.; Min, J.E.; Yoon, S.J.; Kim, H.M.; Yang, E.; Hwang, E.S.; Park, J.H.; Hong, S.-S.; et al. Steroidomics for the Prevention, Assessment, and Management of Cancers: A Systematic Review and Functional Analysis. Metabolites 2019, 9, 199. https://doi.org/10.3390/metabo9100199
Anh NH, Long NP, Kim SJ, Min JE, Yoon SJ, Kim HM, Yang E, Hwang ES, Park JH, Hong S-S, et al. Steroidomics for the Prevention, Assessment, and Management of Cancers: A Systematic Review and Functional Analysis. Metabolites. 2019; 9(10):199. https://doi.org/10.3390/metabo9100199
Chicago/Turabian StyleAnh, Nguyen Hoang, Nguyen Phuoc Long, Sun Jo Kim, Jung Eun Min, Sang Jun Yoon, Hyung Min Kim, Eugine Yang, Eun Sook Hwang, Jeong Hill Park, Soon-Sun Hong, and et al. 2019. "Steroidomics for the Prevention, Assessment, and Management of Cancers: A Systematic Review and Functional Analysis" Metabolites 9, no. 10: 199. https://doi.org/10.3390/metabo9100199
APA StyleAnh, N. H., Long, N. P., Kim, S. J., Min, J. E., Yoon, S. J., Kim, H. M., Yang, E., Hwang, E. S., Park, J. H., Hong, S. -S., & Kwon, S. W. (2019). Steroidomics for the Prevention, Assessment, and Management of Cancers: A Systematic Review and Functional Analysis. Metabolites, 9(10), 199. https://doi.org/10.3390/metabo9100199