Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
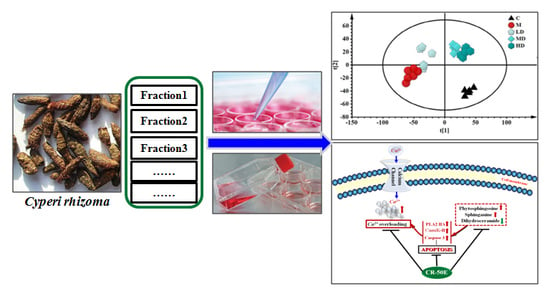
2.2. Preparation of CR Extracts and Sample Quality Control
2.3. Cell Culture
2.4. Corticosterone Damage and Drug Treatments
2.5. Cell Viability Assay
2.6. Lactate Dehydrogenase Assay
2.7. Cell Metabonomics Study
2.7.1. Cell Quenching and Extraction
2.7.2. UPLC-Q-TOF/MS Analysis
2.7.3. Multivariate Statistical Analysis
2.8. Apoptosis Detection
2.9. Measurement of Intracellular Ca2+ Concentration
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. CR-50E Shows the Best Protection Effect for Corticosterone-Induced PC12 Cell Injury among the Fractions of CR
3.2. CR-50E Increases the Survival Rate of the Corticosterone-Induced PC12 Cells
3.3. CR-50E Inhibits Cell Apoptosis Induced by Corticosterone
3.4. CR-50E Reduces Oxidative Stress Induced by Corticosterone
3.5. CR-50E Improves Intracellular Abnormal Sphingolipids Metabolism Induced by Corticosterone
3.6. CR-50E Suppresses the Expressions of PLA2, CaMK II, and Caspase-3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nima, Z.A.; Jabier, M.S.; Wagi, R.I.; Hussain, H.A. Extraction, identification and antibacterial activityof Cyperus oil fromIraqi C. rotundus. Eng. Technol. J. 2008, 26, 1156–1160. [Google Scholar]
- Peerzada, A.M.; Ali, H.H.; Naeem, M.; Latif, M.; Bukhari, A.H.; Tanveer, A. Cyperus rotundus L.: Traditionaluses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2015, 174, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, W.; Li, Z.H.; Qian, D.W.; Guo, J.M.; Shang, E.X.; Su, S.L.; Tang, Y.P.; Duan, J.A. Comparisons of pharmacokinetic and tissue distribution profile of four major bioactive components after oral administration of Xiang-Fu-Si-Wu Decoction effective fraction in normal and dysmenorrheal symptom rats. J. Ethnopharmacol. 2014, 154, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.M.; Yu, M.; Ma, L.Y.; Zhang, H.W.; Zou, Z.M. Chaihu-Shu-Gan-San regulates phospholipids and bile acid metabolism against hepatic injury induced by chronic unpredictable stress in rat. J. Chromatogr. B 2017, 1064, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chen, Y.; Shen, Q.; Guo, X.; Zhang, Y.; Chen, G. Neural Plasticity Associated with Hippocampal PKA-CREB and NMDA Signaling Is Involved in the Antidepressant Effect of Repeated Low Dose of Yueju Pill on Chronic Mouse Model of Learned Helplessness. Neural Plast. 2017, 2017, 9160515. [Google Scholar] [CrossRef]
- Ito, N.; Nagai, T.; Yabe, T.; Nunome, S.; Hanawa, T.; Yamada, H. Antidepressant-like activity of a Kampo (Japanese herbal) medicine, Koso-san (Xiang-Su-San), and its mode of action via the hypothalamic-pituitary-adrenal axis. Phytomedicine 2006, 13, 658–667. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, S.J.; Jun, B.G.; Lee, K.T.; Hong, S.P.; Oh, M.S.; Jang, D.S.; Choi, J.H. α-Cyperone, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced COX-2 expression and PGE2 production through the negative regulation of NFκB signalling in RAW 264.7 cells. J. Ethnopharmacol. 2013, 147, 208–214. [Google Scholar] [CrossRef]
- Lee, C.H.; Hwang, D.S.; Kim, H.G.; Oh, H.; Park, H.; Cho, J.H.; Lee, J.M.; Jang, J.B.; Lee, K.S.; Oh, M.S. Protective effect of Cyperi rhizoma against 6-hydroxydopamine-induced neuronal damage. J. Med. Food 2010, 13, 564–571. [Google Scholar] [CrossRef]
- Kim, H.G.; Hong, J.; Huh, Y.; Park, C.; Hwang, D.S.; Choi, J.H.; Oh, M.S. Cyperi Rhizoma inhibits the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced reduction in nigrostriatal dopaminergenic neurons in estrogen-deprived mice. J. Ethnopharmacol. 2013, 148, 322–328. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Liu, Y.H. Study on Antidepressant Effect and Mechanism by Cyperus rotundus Extracts. Chin. J. ETMF 2012, 18, 191–193. [Google Scholar]
- Wang, J.M.; Ma, Y.X.; Zhang, B.; Li, Q.W.; Cui, Y. Antidepressant-like effects of extracts isolated from rhizomes of Cyperus rotundus, L. Lishizhen Med. Mater. Med. Res. 2013, 24, 779–781. [Google Scholar]
- Li, S.Y.; Xie, Y.L. Effects of volatile oils from Cyperus rotundus on the anxiety behaviors of mice exposed to chronic restraint stress. Chin. Tradit. Pat. Med. 2018, 40, 2140–2143. [Google Scholar]
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y. Neuroprotective effect of polysaccharides from Gastrodia elata blume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mao, Y.S.; Li, C.; Wang, H.; Ji, J.L. Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model. Int. J. Clin. Exp. Pathol. 2014, 7, 4577–4586. [Google Scholar] [PubMed]
- Jiang, Y.; Li, Z.; Liu, Y.; Liu, X.; Chang, Q.; Liao, Y.; Pan, R. Neuroprotective effect of water extract of Panax ginseng on corticosterone-induced apoptosis in PC12 cells and its underlying molecule mechanisms. J. Ethnopharmacol. 2015, 159, 102–112. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, Y.M.; Liu, Y.M.; Guo, Z.; Shen, S.N.; Liu, X.M.; Pan, R.L. Saikosaponin D acts against corticosterone-induced apoptosis via regulation of mitochondrial GR translocation and a GR-dependent pathway. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 53, 80–89. [Google Scholar] [CrossRef]
- Fillet, M.; Frédérich, M. The emergence of metabolomics as a key discipline in the drug discovery process. Drug Discov. Today Technol. 2015, 13, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Dai, W.; Li, Y. Neuroprotective effect of a physiological ratio of testosterone and estradiol on corticosterone-induced apoptosis in PC12 cells via Traf6/TAK1 pathway. Toxicol. In Vitro 2018, 50, 257–263. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Li, X.; Gong, W.X.; Tian, J.S.; Gao, X.X.; Gao, L.; Zhang, X.; Du, G.H.; Qin, X.M. Protective effect of isoliquiritin against corticosterone-induced neurotoxicity in PC12 cells. Food Funct. 2017, 8, 1235–1244. [Google Scholar] [CrossRef]
- Choi, J.E.; Park, D.M.; Chun, E.; Choi, J.J.; Seo, J.H.; Kim, S.; Son, J.; Do, M.; Kim, S.Y.; Park, Y.C.; et al. Control of stress-induced depressive disorders by So-ochim-tang-gamibang, a Korean herbal medicine. J. Ethnopharmacol. 2017, 196, 141–150. [Google Scholar] [CrossRef]
- He, X.Y.; Chen, J.L.; Xiang, H.; Gao, Y.; Tian, J.S.; Qin, X.M.; Du, G.H. Comparative study of the corticosterone and glutamate induced PC12 cells depression model by 1H NMR metabolomics. Yao Xue Xue Bao 2017, 52, 245–252. [Google Scholar] [PubMed]
- Nihar, K.; Kaushik, S.; Thomas, J.A.; Paul, C.T. Passage Variation of PC12 Cells Results in Inconsistent Susceptibility to Externally Induced Apoptosis. ACS Chem. Neurosci. 2017, 8, 82–88. [Google Scholar]
- Halama, A. Metabolomics in cell culture—A strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch Biochem. Biophys. 2014, 564, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Jia, H.M.; Ma, L.Y.; Yu, M.; Yang, Y.; Liu, Y.; Zhang, H.W.; Zou, Z.M. Metabolic profiling of hypoxia/reoxygenation injury in H9c2 cells reveals the accumulation of phytosphingosine and the vital role of Dan-Shen in Xin-Ke-Shu. Phytomedicine 2018, 49, 83–94. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Abbineni, P.S.; Coorssen, J.R. Sphingolipids modulate docking, Ca2+ sensitivity and membrane fusion of native cortical vesicles. Int. J. Biochem. Cell Biol. 2018, 104, 43–54. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhou, C.; Jia, H.M.; Chang, X.; Zou, Z.M. Standardized Chinese Formula Xin-Ke-Shu inhibits the myocardium Ca(2+) overloading and metabolic alternations in isoproterenol-induced myocardial infarction rats. Sci. Rep. 2016, 6, 30208. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2015, 1219, 1–9. [Google Scholar]
- Patwardhan, G.A.; Liu, Y.Y. Sphingolipids and expression regulation of genes in cancer. Prog. Lipid Res. 2011, 50, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Corbacho, M.J.; Salama, M.F.; Canals, D.; Senkal, C.E.; Obeid, L.M. Sphingolipids in mitochondria. Biochim. Biophys. Acta 2017, 1862, 56–68. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.; Her, Y.; Seong, I.; Park, S.; Bhattarai, D.; Jin, G.; Lee, K.; Chung, G.; Hwang, S.; et al. Phytosphingosine promotes megakaryocytic differentiation of myeloid leukemia cells. BMB Rep. 2015, 48, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Park, M.T.; Kim, M.J.; Kang, Y.H.; Choi, S.Y.; Lee, J.H.; Choi, J.A.; Kang, C.M.; Cho, C.K.; Kang, S.; Bae, S.; et al. Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells through ROS-dependent and -independent AIF release. Blood 2005, 105, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Gagliostro, V.; Casas, J.; Caretti, A.; Abad, J.L.; Tagliavacca, L.; Ghidoni, R.; Fabrias, G.; Signorelli, P. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 2012, 44, 2135–2143. [Google Scholar] [CrossRef] [PubMed]





| NO. | Rt(min) | m/z | Metabolite | VIP | M/C a | CR-50E/M b |
|---|---|---|---|---|---|---|
| CE1 | 5.32 | 274.2749 | Unidentified | 2.01 | ↓ ** | ↑ ** |
| CE2 | 5.35 | 318.3011 | phytosphingosine | 3.93 | ↑ ** | ↓ ** |
| CE3 | 5.37 | 362.3268 | dihydroceramide | 2.24 | ↓ ** | ↑ ** |
| CE4 | 5.91 | 302.3058 | Sphinganine | 2.21 | ↑ ** | ↓ ** |
| CE5 | 6.20 | 265.1473 | 5β-cyprinol sulfate | 3.59 | ↓ ** | ↑ ** |
| CE6 | 6.78 | 309.1732 | Unidentidied | 2.32 | ↓ ** | ↑ ** |
| CE7 | 7.13 | 478.2930 | LysoPE(18:2(9Z,12Z)/0:0) | 1.05 | ↓ ** | ↑ ** |
| CE8 | 7.16 | 506.3243 | LysoPE(0:0/20:2(11Z,14Z)) | 1.29 | ↓ ** | ↑ ** |
| CE9 | 7.64 | 653.3003 | DG (36:5) | 1.41 | ↓ ** | ↑ * |
| CE10 | 7.99 | 480.3085 | LysoPE(0:0/18:1(11Z)) | 1.09 | ↑ ** | ↓ ** |
| CE11 | 8.02 | 508.3398 | LysoPC(P-18:0) | 1.22 | ↑ ** | ↓ ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Liu, Y.; Yu, M.; Shang, H.; Zhang, H.; Ma, L.; Zhang, T.; Zou, Z. Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading. Metabolites 2019, 9, 244. https://doi.org/10.3390/metabo9110244
Jia H, Liu Y, Yu M, Shang H, Zhang H, Ma L, Zhang T, Zou Z. Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading. Metabolites. 2019; 9(11):244. https://doi.org/10.3390/metabo9110244
Chicago/Turabian StyleJia, Hongmei, Yang Liu, Meng Yu, Hai Shang, Hongwu Zhang, Liyan Ma, Tao Zhang, and Zhongmei Zou. 2019. "Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading" Metabolites 9, no. 11: 244. https://doi.org/10.3390/metabo9110244
APA StyleJia, H., Liu, Y., Yu, M., Shang, H., Zhang, H., Ma, L., Zhang, T., & Zou, Z. (2019). Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading. Metabolites, 9(11), 244. https://doi.org/10.3390/metabo9110244