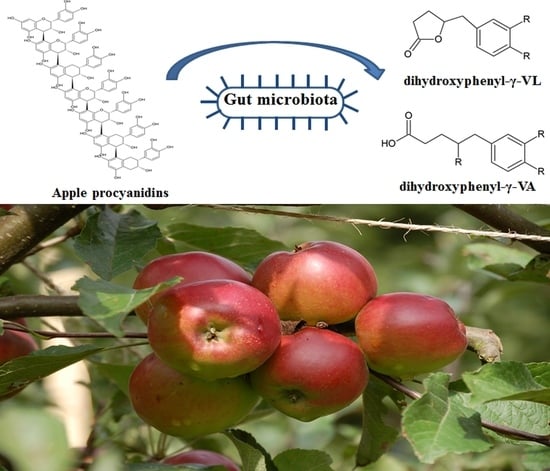
Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples
Abstract
:1. Introduction
2. Results
2.1. Flavan-3-ol and PAC Composition of Study Apples
2.2. Targeted Analysis of Free and Conjugated PVLs and PVAs in Urine
2.3. Bioavailability and Quantitative Determination of Conjugated PVL and PVA
2.4. The Ratio of Sulfate/Glucuronide Conjugates of 5-(3′,4′-dihydroxyphenyl)-γ-VL Suggests the Presence of Phase II Metabotypes
2.5. Untargeted Analysis of Conjugated (–)-Epicatechin
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Dietary Intervention and Sample Collection
4.3. Sample Preparation for PVL Quantitation
4.4. Targeted UHPLC-ESI-QqQ-MS
4.5. Sample Preparation for Untargeted Analysis and Targeted Selection of Epicatechin Phase II Metabolites
4.6. Determination of Flavan-3-Ols Content in Study Apples
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solicitation of Written Comments on Proposed Definition of Bioactive Food Component. Available online: https://www.govinfo.gov/content/pkg/FR-2004-09-16/pdf/04-20892.pdf (accessed on 14 October 2019).
- Hollman, P.C.; Arts, I.C.; Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macià, A.; Romero, M.-P.; Salvadó, M.-J.; Bustos, M.; Fernández-Larrea, J.; Motilva, M.-J. Determination of procyanidins and their metabolites in plasma samples by improved liquid chromatography–tandem mass spectrometry. J. Chromatogr. 2009, 877, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andres-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography–tandem mass spectrometry. J. Chromatogr. 2009, 1216, 7258–7267. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Garrido, I.; Monagas, M.; Gòmez-Cordovés, C.; Medina-Remòn, A.; Andrès-Lacueva, C.; Bartolomé, B. Profile of Plasma and Urine Metabolites after the Intake of Almond [Prunus dulcis (Mill.) D.A. Webb] Polyphenols in Humans. J. Agric. Food Chem. 2009, 57, 10134–10142. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Hooper, L.; Kay, C.; Abdelhamid, A.; A Kroon, P.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.C.; Spencer, J.P.E.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- González-Sarrías, A.; Combet, E.; Pinto, P.; Mena, P.; Dall’Asta, M.; Aloy, M.G.; Rodriguez-Mateos, A.; Gibney, E.R.; Dumont, J.; Massaro, M.; et al. A systematic review and meta-analysis of the effects of flavanol-containing tea, cocoa and apple products on body composition and blood lipids: Exploring the factors responsible for variability in their efficacy. Nutrients 2017, 9, 746. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Heiss, C.; Spencer, J.P.; Kelm, M.; Schroeter, H. Recommending flavanols and procyanidins for cardiovascular health: Revisited. Mol. Asp. Med. 2018, 61, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the Modification of the Authorisation of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium-Dependent Vasodilation Pursuant to Article 13(5) of Regulation (EC) No 1924/2006 Following a Request in Accordan. EFSA J. 2014, 12. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3654 (accessed on 14 October 2019).
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free. Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodmann, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-Rich Apple Improves Endothelial Function in Individuals at Risk for Cardiovascular Disease: A Randomized Controlled Clinical Trial. Mol. Nutr. Food Res. 2018, 62, 3. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is There Room for Improving the Nutraceutical Composition of Apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Cordero, C.E.I.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mateos, A.; Cifuentes-Gomez, T.; Gonzalez-Salvador, I.; Ottaviani, J.I.; Schroeter, H.; Kelm, M.; Heiss, C.; Spencer, J.P.E.; Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; et al. Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. Mol. Nutr. Food Res. 2015, 59, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.-P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Fong, R.; Kimball, J.; Ensunsa, J.L.; Britten, A.; Lucarelli, D.; Luben, R.; Grace, P.B.; Mawson, D.H.; Tym, A.; et al. Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies. Sci. Rep. 2018, 8, 9859. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef]
- Van Der Hooft, J.J.J.; De Vos, R.C.H.; Mihaleva, V.; Bino, R.J.; Ridder, L.; De Roo, N.; Jacobs, D.M.; Van Duynhoven, J.P.M.; Vervoort, J. Structural Elucidation and Quantification of Phenolic Conjugates Present in Human Urine after Tea Intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-14C] (−)-epicatechin in humans: Implications for the assessment of efficacy, safety and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef]
- Brindani, N.; Mena, P.; Calani, L.; Benzie, I.; Choi, S.-W.; Brighenti, F.; Zanardi, F.; Curti, C.; Del Rio, D.; Choi, S. Synthetic and analytical strategies for the quantification of phenyl-γ-valerolactone conjugated metabolites in human urine. Mol. Nutr. Food Res. 2017, 61, 1700077. [Google Scholar] [CrossRef]
- Trôst, K.; Ulaszewska, M.M.; Stanstrup, J.; Albanese, D.; De Filippo, C.; Tuohy, K.M.; Natella, F.; Scaccini, C.; Mattivi, F. Host: Microbiome co-metabolic processing of dietary polyphenols – An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects. Food Res. Int. 2018, 112, 108–128. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.M.; Trost, K.; Stanstrup, J.; Tuohy, K.M.; Franceschi, P.; Chong, M.F.-F.; George, T.; Minihane, A.M.; Lovegrove, J.A.; Mattivi, F. Urinary metabolomic profiling to identify biomarkers of a flavonoid-rich and flavonoid-poor fruits and vegetables diet in adults: The FLAVURS trial. Metabolomics 2016, 12, 32. [Google Scholar] [CrossRef]
- Curti, C.; Brindani, N.; Battistini, L.; Sartori, A.; Pelosi, G.; Mena, P.; Brighenti, F.; Zanardi, F.; Del Rio, D. Catalytic, Enantioselective Vinylogous Mukaiyama Aldol Reaction of Furan-Based Dienoxy Silanes: A Chemodivergent Approach to γ-Valerolactone Flavan-3-ol Metabolites and δ-Lactone Analogues. Adv. Synth. Catal. 2015, 357, 4082–4092. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C.; Rodriguez-Mateos, A. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauzour, D.; Rodriguez-Ramiro, I.; Rushbrook, S.; Ipharraguerre, I.R.; Bevan, D.; Davies, S.; Tejera, N.; Mena, P.; De Pascual-Teresa, S.; Del Rio, D.; et al. n-3 Fatty acids combined with flavan-3-ols prevent steatosis and liver injury in a murine model of NAFLD. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Van Der Hooft, J.J.J.; Van Dorsten, F.A.; Peters, S.; Foltz, M.; Gomez-Roldan, V.; Vervoort, J.; De Vos, R.C.H.; Jacobs, D.M. Rapid and Sustained Systemic Circulation of Conjugated Gut Microbial Catabolites after Single-Dose Black Tea Extract Consumption. J. Proteome Res. 2014, 13, 2668–2678. [Google Scholar] [CrossRef]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef]
- Li, C.; Lee, M.-J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.-T.; et al. Structural Identification of Two Metabolites of Catechins and Their Kinetics in Human Urine and Blood after Tea Ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Van Velzen, E.J.J.; Westerhuis, J.A.; Grün, C.H.; Jacobs, D.M.; Eilers, P.H.C.; Mulder, T.P.; Foltz, M.; Garczarek, U.; Kemperman, R.; Vaughan, E.E.; et al. Population-based nutrikinetic modeling of polyphenol exposure. Metabolomics 2014, 10, 1059–1073. [Google Scholar] [CrossRef]
- Miners, J.O.; A McKinnon, R.; I MacKenzie, P. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology 2002, 181, 453–456. [Google Scholar] [CrossRef]
- Koster, H.; Halsema, I.; Scholtens, E.; Knippers, M.; Mulder, G.J. Dose-dependent shifts in the sulfation and glucuronidation of phenolic compounds in the rat in vivo and in isolated hepatocytes. Biochem. Pharmacol. 1981, 30, 2569–2575. [Google Scholar] [CrossRef]
- Bolca, S.; Possemiers, S.; Maervoet, V.; Huybrechts, I.; Heyerick, A.; Vervarcke, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; De Henauw, S.; et al. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: A dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br. J. Nutr. 2007, 98, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Summer, S.; King, E.C.; Heubi, J.E.; Cole, S.; Guy, T.; Hokin, B. Dietary factors influence production of the soy isoflavone metabolite s-(-)equol in healthy adults. J. Nutr. 2013, 143, 1950–1958. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ulaszewska, M.; Mattivi, F.; Del Bubba, M. Untargeted Metabolomics Analytical Strategy Based on Liquid Chromatography/Electrospray Ionization Linear Ion Trap Quadrupole/Orbitrap Mass Spectrometry for Discovering New Polyphenol Metabolites in Human Biofluids after Acute Ingestion of Vaccinium myrti. J. Am. Soc. Mass Spectrom. 2019, 30, 381–402. [Google Scholar] [CrossRef]
- Chambers, M.C.; MacLean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Proanthocyanidin profile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chem. 2011, 126, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Haug, K.; Salek, R.M.; Conesa, P.; Hastings, J.; de Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P.; et al. MetaboLights—An open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2013, 41, D781–D786. [Google Scholar] [CrossRef] [PubMed]





| Flavan-3-ol | Content (μmol) |
|---|---|
| Free (+)-catechin | 86.57 ± 4.68 |
| Free (−)-epicatechin | 688.85 ± 29.96 |
| Free gallocatechin | 0.17 ± 0.03 |
| Free epigallocatechin | 0.00 ± 0.00 |
| Free catechin gallate | 0.18 ± 0.00 |
| Procyanidin B1 | 176.35 ± 11.90 |
| Procyanidin B2 | 1882.18 ± 62.13 |
| Total PACs | 2922.50 ± 121.74 |
| PAC mDP | 8.5 ± 0.01 |
| Total flavan-3-ols | 5756.81 |
| Metabolite | ID | Transition (m/z) | Retention Time (min) |
|---|---|---|---|
| 5-phenyl-γ-VL-3′-sulfate | 1 | 271 > 191 | 2.88 |
| 5-(3′-hydroxyphenyl)-γ-VL-4′-sulfate | 2 | 287 > 207 | 2.66 |
| 5-phenyl-γ-VL-3′-glucuronide | 3 | 367 > 191 | 2.10 |
| 5-(hydroxyphenyl)-γ-VL-glucuronide (3 ′,4 ′ isomer) | 4 | 383 > 207 | 1.76 |
| 5-phenyl-γ-VL-methoxy-sulfate 1 | 5 | 301 > 206 | 2.65 |
| 5-phenyl-γ-VL-methoxy-sulfate 2 | 6 | 301 > 206 | 2.83 |
| 5-phenyl-γ-VL-sulfate-glucuronide | 7 | 463 > 207 | 2.05 |
| 5-(phenyl)-γ-VL-methoxy-glucuronide | 8 | 397 > 221 | 2.03 |
| 4-hydroxy-5-(hydroxyphenyl)-VA-sulfate | 9 | 305 > 225 | 2.16 |
| 4-hydroxy-5-(hydroxyphenyl)-VA-glucuronide | 10 | 401 > 225 | 2.05 |
| 4-hydroxy-5-phenyl-VA-methoxy-sulfate | 11 | 319 > 239 | 2.12 |
| PVL and PVA Metabolites | Cumulative Excretion (μmol) and Relative % of Different Metabolites in the 11 Study Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S9 | S4 | S3 | S5 | S8 | S2 | S1 | S10 | S6 | S11 | S7 | ACE | |
| 5-phenyl-γ-VL-3′-sulfate | 8.61 (1.6) | 10.85 (2.7) | 2.43 0.8) | 2.95 (1.4) | 0.56 (0.3) | 8.33 (7.7) | 2.17 (1.9) | 24.09 (20.9) | 7.02 (9.9) | 53.23 (43.3) | 3.31 (4.5) | 11.23 (±15.38) |
| 5-(3′-hydroxyphenyl)-γ-VL-4′-sulfate | 192.05 (36.6) | 174.55 (42.9) | 168.04 (58.1) | 104.29 (50.6) | 91.97 (47.6) | 68.18 (62.7) | 79.06 (70.1) | 66.24 (57.58) | 40.21 (56.9) | 31.41 (25.6) | 57.79 (77.8) | 96.92 (±56.92) |
| 5-phenyl-γ-VL-3′-glucuronide | 6.27 (1.2) | 4.26 (1.0) | 0.03 (0.0) | 0.42 (0.2) | 0.04 (0.0) | 0.04 (0.0) | 0.05 (0.0) | 2.76 (2.4) | 3.70 (5.2) | 25.60 (20.8) | 0.04 (0.1) | 3.93 (±7.51) |
| 5-(hydroxyphenyl)-γ-VL-glucuronide | 275.60 (52.6) | 174.40 (42.9) | 93.17 (32.2) | 83.63 (40.6) | 81.44 (42.2) | 23.81 (21.9) | 19.54 (17.3) | 16.61 (14.4) | 9.93 (14.1) | 6.91 (5.6) | 4.82 (6.5) | 71.81 (±85.64) |
| 5-phenyl-γ-VL-methoxy-sulfate (1) | 0.44 (0.1) | 0.52 (0.1) | 0.46 (0.2) | 0.39 (0.2) | 0.36 (0.2) | 0.34 (0.3) | 0.31 (0.3) | 0.34 (0.3) | 0.23 (0.3) | 0.39 (0.3) | 0.29 (0.4) | 0.37 (±0.08) |
| 5-phenyl-γ-VL-methoxy-sulfate (2) | 1.30 (0.2) | 1.00 (0.2) | 1.11 (0.4) | 0.84 (0.4) | 0.57 (0.3) | 0.59 (0.5) | 0.59 (0.5) | 0.50 (0.4) | 0.33 (0.5) | 0.36 (0.3) | 0.33 (0.4) | 0.68 (±0.33) |
| 5-phenyl-γ-VL-methoxy-glucuronide | 1.96 (0.4) | 1.18 (0.3) | 0.80 (0.3) | 0.81 (0.4) | 1.07 (0.6) | 0.62 (0.6) | 0.81 (0.7) | 1.40 (1.2) | 0.30 (0.4) | 0.43 (0.6) | 0.39 (0.5) | 0.89 (±0.49) |
| 5-phenyl-γ-VL-sulfate-glucuronide | 30.91 (5.9) | 33.92 (8.3) | 12.30 (4.3) | 10.67 (5.2) | 9.57 (5.0) | 6.83 (6.3) | 5.51 (4.9) | 3.10 (2.7) | 6.87 (9.7) | 3.20 (2.6) | 5.49 (7.4) | 11.67 (±10.67) |
| 4-hydroxy-5-(hydroxyphenyl)-VA-sulfate | 7.18 (1.4) | 6.14 (1.5) | 10.60 (3.7) | 2.07 (1.0) | 7.39 (3.8) | <LOD (0.0) | 4.67 (4.1) | 0.06 (0.1) | 1.85 (2.6) | 0.30 (0.2) | 1.82 (2.4) | 3.82 (±3.58) |
| 4-hydroxy-5-(hydroxyphenyl)-VA-methoxy-sulfate | 0.02 (0.0) | 0.01 (0.0) | 0.04 (0.0) | 0.01 (0.0) | 0.02 (0.0) | <LOD (0.0) | <LOD (0.0) | <LOD (0.0) | 0.02 (0.0) | 0.01 (0.0) | 0.01 (0.0) | 0.01 (±0.01) |
| 4-hydroxy-5-(hydroxyphenyl)-VA-glucuronide | 0.02 (0.0) | 0.17 (0.0) | 0.21 (0.1) | 0.02 (0.0) | 0.04 (0.0) | 0.02 (0.0) | 0.03 (0.0) | 0.02 (0.0) | 0.21 (0.3) | 0.98 (0.8) | 0.02 (0.0) | 0.16 (±0.28) |
| SUM (μmol) | 524.35 | 406.98 | 289.18 | 206.10 | 193.03 | 108.77 | 112.73 | 115.13 | 70.68 | 122.79 | 74.30 | 202.19 (±147.53) |
| % excretion (to all flavan-3-ols) | 9.1 | 7.1 | 5.0 | 3.6 | 3.4 | 1.9 | 2.0 | 2.0 | 1.2 | 2.1 | 1.3 | 3.52 (±2.56) |
| % excretion (to total PACs) | 17.9 | 13.9 | 9.9 | 7.0 | 6.6 | 3.7 | 3.9 | 3.9 | 2.4 | 4.2 | 2.5 | 6.90 (±5.04) |
| Subject | Ratio 5-Hydroxyphenyl-γ-VL Sulfate/Glucuronide | Cumulative Excretion (μmol) | Ratio Epicatechin Sulfate/Glucuronide |
|---|---|---|---|
| S1 | 4.0 | 112.73 | 3.7 |
| S2 | 2.9 | 108.77 | 7.2 |
| S3 | 1.8 | 289.18 | 6.8 |
| S4 | 1.0 | 406.98 | 5.8 |
| S5 | 1.2 | 206.10 | 6.7 |
| S6 | 4.0 | 70.68 | 3.9 |
| S7 | 12.0 | 74.30 | 8.9 |
| S8 | 1.1 | 193.03 | 2.5 |
| S9 | 0.7 | 524.35 | 2.3 |
| S10 | 4.0 | 115.13 | 3.6 |
| S11 | 4.5 | 122.79 | 4.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anesi, A.; Mena, P.; Bub, A.; Ulaszewska, M.; Del Rio, D.; Kulling, S.E.; Mattivi, F. Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples. Metabolites 2019, 9, 254. https://doi.org/10.3390/metabo9110254
Anesi A, Mena P, Bub A, Ulaszewska M, Del Rio D, Kulling SE, Mattivi F. Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples. Metabolites. 2019; 9(11):254. https://doi.org/10.3390/metabo9110254
Chicago/Turabian StyleAnesi, Andrea, Pedro Mena, Achim Bub, Marynka Ulaszewska, Daniele Del Rio, Sabine E. Kulling, and Fulvio Mattivi. 2019. "Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples" Metabolites 9, no. 11: 254. https://doi.org/10.3390/metabo9110254
APA StyleAnesi, A., Mena, P., Bub, A., Ulaszewska, M., Del Rio, D., Kulling, S. E., & Mattivi, F. (2019). Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples. Metabolites, 9(11), 254. https://doi.org/10.3390/metabo9110254