Study of the Differentially Expressed Genes in the Pomacea canaliculata Transcriptome after Treatment with Pedunsaponin A
Abstract
:1. Introduction
2. Results
2.1. Results of RNA-Seq and Sequence Shearing
2.2. The Results of De Novo Assembly
2.3. Functional Annotation of P. canaliculata Unigenes
2.3.1. The Results of GO Annotation
2.3.2. The Results of KEGG Annotation
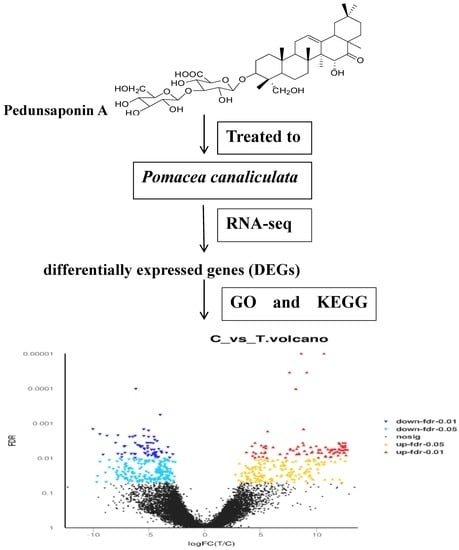
2.4. The Analyses of DEGs
2.4.1. GO Enrichment Analyses of DEGs
2.4.2. The KEGG Pathway Annotation Results of the DEGs
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Sample Collection and RNA Extraction
4.2.2. RNA-Seq
4.2.3. Sequence Shearing and de Novo Assembly
4.2.4. Annotation of Unigene Functions
4.2.5. Screening and Analysis of DEGs
Author Contributions
Funding
Conflicts of Interest
References
- Yang, C.P.; Zhang, M.; Lei, B.; Gong, G.S.; Yue, G.Z.; Chang, X.L.; Sun, X.F.; Tian, Y.; Chen, H.B. Active saponins from root of Pueraria peduncularis (Grah. ex Benth.) Benth. and their molluscicidal effects on Pomacea canaliculata. Pest Manag. Sci. 2017, 73, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Tian, Y.; Lv, T.X.; Chang, X.L.; Zhang, M.; Gong, G.S.; Zhao, L.L.; Yang, S.; Chen, H.B. Histopathological effects of pedunsaponin A on Pomacea canaliculata. Pestic. Biochem. Physiol. 2018, 148, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Lv, T.X.; Wang, B.; Qiu, X.Y.; Luo, L.Y.; Zhang, M.; Yue, G.Z.; Qin, G.W.; Xie, D.S.; Chen, H.B. The damaging effects of pedunsaponin A on Pomacea canaliculata hemocytes. Toxins 2019, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Geng, T. Study on Gene Expression of Mebendazole Against Gyradactylus kobayashii by RNA-seq. Master’s thesis, Northwest Agriculture and Forestry University, Yangling, China, 2016. [Google Scholar]
- Hsu, L.S.; Chiou, B.H.; Hsu, T.W.; Wang, C.C.; Chen, S.C. The Regulation of Transcriptome Responses in Zebrafish Embryo Exposure to Triadimefon. Environ. Toxicol. 2017, 32, 217–226. [Google Scholar] [CrossRef]
- Wei, L.B.; Miao, H.M.; Zhang, H.Y. Transcriptomic Analysis of Sesame Development. Sci. Agric. Sin. 2012, 45, 1246–1256. [Google Scholar]
- Ding, Y.; Jones, G.M.; Brimacombe, C.; Uchida, K.; Aizawa, S.; Logan, S.M.; Kelly, J.F.; Jarrell, K.F. Identification of a gene involved in the biosynthesis pathway of the terminal sugar of the archaellin N-linked tetrasaccharide in Methanococcus maripaludis. Antonie van Leeuwenhoek 2016, 109, 131–148. [Google Scholar] [CrossRef]
- Yu, Z.M.; He, C.M.; Silva, J.A.T.D.; Zhang, G.H.; Dong, W.; Luo, J.P.; Duan, J. Molecular cloning and functional analysis of DoUGE related to water-soluble polysaccharides from Dendrobium officinale with enhanced abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2017, 131, 579–599. [Google Scholar] [CrossRef]
- Maron, B.A.; Zhang, Y.Y.; Handy, D.E.; Beuve, A.; Tang, S.S.; Loscalzo, J.; Leopold, J.A. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 7665–7672. [Google Scholar] [CrossRef]
- Secca, T.; Sciaccaluga, M.; Marra, A.; Barberini, L.; Bicchierai, M.C. Biochemical activity and multiple locations of particulate guanylate cyclase in Rhyacophila dorsalis acutidens (Insecta: Trichoptera) provide insights into the cGMP signalling pathway in Malpighian tubules. J. Insect Physiol. 2011, 57, 521–528. [Google Scholar] [CrossRef]
- Li, J.P.; Qin, G.X.; Ren, Y.L.; Zhang, Q.L.; Wang, D.L.; Sun, B.X.; Zhao, Z.H.; Jiang, H.L. Effect of safflower seed oil on liver transcriptome of finishing pigs based on high-throughput RNA-seq technology. Chin. J. Vet. Sci. 2015, 35, 1293–1301. [Google Scholar]
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011, 12, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zong, H.; Yu, R.T.; Han, X.L.; Zheng, D.Y.; Han, L. Proteomics study of mature immune cell phagocytes. Chin. J. Cell. Mol. Immunol. 2010, 26, 833–835. [Google Scholar]
- Zhao, S.Q.; Liu, J.H. Role of Rab GTPases in phagosome maturation. J. Med. Mol. Biol. 2017, 14, 360–364. [Google Scholar]
- Ding, L.; Ni, Y.H.; Hu, Q.G.; Hou, Y.Y. cGAS-STING: The novel mechanism of cytosolic DNA sensing pathways. Prog. Biochem. Biophys. 2014, 41, 830–838. [Google Scholar]
- Han, J.; Xu, G.; Xu, T. The miiuy croaker microRNA transcriptome and microRNA regulation of RIG-I like receptor signaling pathway after poly (I:C) stimulation. Fish Shellfish Immunol. 2016, 54, 419–426. [Google Scholar] [CrossRef]
- Jung, M.; Dritschilo, A. NF-kappa B signaling pathway as a target for human tumor radiosensitization. Semin. Radiat. Oncol. 2001, 11, 346–351. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef]
- Cock, P.J.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequence with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RESM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]








| Sample Name | Original Sequence Number | Nonredundant Sequence Number | Error% | Q20% | Q30% | GC% |
|---|---|---|---|---|---|---|
| C_1 | 83351566 | 80289528 | 0.0149 | 96.96 | 92.1 | 45.47 |
| C_2 | 71588586 | 69052548 | 0.0148 | 96.99 | 92.14 | 46.94 |
| C_3 | 65875322 | 63312812 | 0.015 | 96.94 | 91.99 | 47.55 |
| T_1 | 74871166 | 72325846 | 0.0146 | 97.07 | 92.35 | 45.26 |
| T_2 | 68095074 | 65957654 | 0.0144 | 97.17 | 92.61 | 45.13 |
| T_3 | 77424610 | 74869170 | 0.0144 | 97.14 | 92.54 | 45.55 |
| Average | 73534387 | 70967926 | 0.0147 | 97.05 | 92.29 | 45.98 |
| Total | 441206324 | 425807558 | — | — | — | — |
| Type | Total Number | Base Number | GC (%) | Average Length (bp) | N50 (bp) |
|---|---|---|---|---|---|
| transcripts | 89,638 | 184,800,908 | 42.38 | 2061.64 | 3385 |
| unigenes | 48,567 | 85,118,502 | 42.23 | 1752.6 | 3135 |
| Database | Total Number of Unigenes | Annotated Unigenes | Percentage (%) |
|---|---|---|---|
| Kyoto Encyclopedia of Genes and Genomes (KEGG) | 48,567 | 8751 | 18.02 |
| Gene Ontology (GO) | 48,567 | 6020 | 12.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Lv, T.; Zhang, Y.; Wang, B.; Zhao, X.; Zhang, M.; Gong, G.; Chang, X.; Yue, G.; Qiu, X.; et al. Study of the Differentially Expressed Genes in the Pomacea canaliculata Transcriptome after Treatment with Pedunsaponin A. Metabolites 2019, 9, 268. https://doi.org/10.3390/metabo9110268
Yang C, Lv T, Zhang Y, Wang B, Zhao X, Zhang M, Gong G, Chang X, Yue G, Qiu X, et al. Study of the Differentially Expressed Genes in the Pomacea canaliculata Transcriptome after Treatment with Pedunsaponin A. Metabolites. 2019; 9(11):268. https://doi.org/10.3390/metabo9110268
Chicago/Turabian StyleYang, Chunping, Tianxing Lv, Yangyang Zhang, Bin Wang, Xiaomin Zhao, Min Zhang, Guoshu Gong, Xiaoli Chang, Guizhou Yue, Xiaoyan Qiu, and et al. 2019. "Study of the Differentially Expressed Genes in the Pomacea canaliculata Transcriptome after Treatment with Pedunsaponin A" Metabolites 9, no. 11: 268. https://doi.org/10.3390/metabo9110268
APA StyleYang, C., Lv, T., Zhang, Y., Wang, B., Zhao, X., Zhang, M., Gong, G., Chang, X., Yue, G., Qiu, X., Luo, L., & Chen, H. (2019). Study of the Differentially Expressed Genes in the Pomacea canaliculata Transcriptome after Treatment with Pedunsaponin A. Metabolites, 9(11), 268. https://doi.org/10.3390/metabo9110268