Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity
Abstract
:1. Introduction
2. Results
2.1. High Throughput Metabolomic Studies of Plasma Filtrates Using MSI-CE-MS
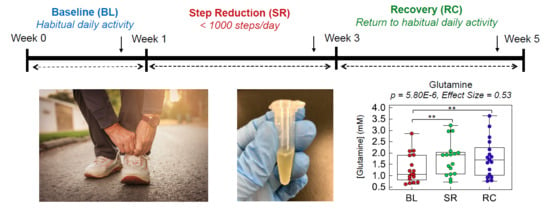
2.2. Plasma Metabolic Phenotyping of Healthy Seniors in a Step Reduction Study
2.3. Evaluating the Effects Short-Term/Acute Physical Inactivity in Older Pre-Diabetic Adults
3. Discussion
4. Materials and Methods
4.1. Study Cohort and Intervention
4.2. Plasma Sample Preparation
4.3. Nontargeted Metabolite Profiling of Plasma Filtrates by MSI-CE-MS
4.4. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Maria Martone, A.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An overview on current definitions, diagnosis and treatment. Curr. Protein Pept. Sci. 2017, 19, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the biological substrate of physical frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Verri, M. Sarcopenic obesity: An appraisal of the current status of knowledge and management in elderly people. J. Nutr. Health Aging 2016, 20, 780–788. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Anker, S.D.; Von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; Marzetti, E. Biomarkers for physical frailty and sarcopenia: State of the science and future developments. J. Cachexia Sarcopenia Muscle 2015, 6, 278–286. [Google Scholar] [CrossRef]
- Cesari, M.; Fielding, R.A.; Pahor, M.; Goodpaster, B.; Hellerstein, M.; van Kan, G.A.; Anker, S.D.; Rutkove, S.; Vrijbloed, J.W.; Isaac, M.; et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. J. Am. Med. Assoc. 1999, 281, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. J. Am. Med. Assoc. 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef]
- McGlory, C.; Von Allmen, M.T.; Stokes, T.; Morton, R.W.; Hector, A.J.; Lago, B.A.; Raphenya, A.R.; Smith, B.K.; McArthur, A.G.; Steinberg, G.R.; et al. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 weeks of physical inactivity in overweight, prediabetic older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1070–1077. [Google Scholar] [CrossRef]
- Kuehnbaum, N.L.; Gillen, J.B.; Kormendi, A.; Lam, K.P.; Dibattista, A.; Gibala, M.J.; Britz-Mckibbin, P. Multiplexed separations for biomarker discovery in metabolomics: Elucidating adaptive responses to exercise training. Electrophoresis 2015, 36, 2226–2236. [Google Scholar] [CrossRef]
- DiBattista, A.; McIntosh, N.; Lamoureux, M.; Al-Dirbashi, O.Y.; Chakraborty, P.; Britz-McKibbin, P. Temporal signal pattern recognition in mass spectrometry: A method for rapid identification and accurate quantification of biomarkers for inborn errors of metabolism with quality assurance. Anal. Chem. 2017, 89, 8112–8121. [Google Scholar] [CrossRef]
- Nori de Macedo, A.; Mathiaparanam, S.; Brick, L.; Keenan, K.; Gonska, T.; Pedder, L.; Hill, S.; Britz-McKibbin, P. The sweat metabolome of screen-positive cystic fibrosis infants: Revealing mechanisms beyond impaired chloride transport. ACS Cent. Sci. 2017, 3, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Saoi, M.; Percival, M.; Nemr, C.; Li, A.; Gibala, M.; Britz-McKibbin, P. Characterization of the human skeletal muscle metabolome for elucidating the mechanisms of bicarbonate ingestion on strenuous interval exercise. Anal. Chem. 2019, 91, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.; Ly, R.; Britz-McKibbin, P. Robust method for high throughput screening of fatty acids by multisegment injection-nonaqueous capillary electrophoresis-mass spectrometry with stringent quality control. Anal. Chem. 2019, 91, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- DiBattista, A.; Rampersaud, D.; Lee, H.; Kim, M.; Britz-McKibbin, P. High throughput screening method for systematic surveillance of drugs of abuse by multisegment injection-capillary electrophoresis-mass spectrometry. Anal. Chem. 2017, 89, 11853–11891. [Google Scholar] [CrossRef] [PubMed]
- Kuehnbaum, N.L.; Kormendi, A.; Britz-McKibbin, P. Multisegment injection-capillary electrophoresis-mass spectrometry: A high-throughput platform for metabolomics with high data fidelity. Anal. Chem. 2013, 85, 10664–10669. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Yamamoto, M.; Pinto-Sanchez, M.I.; Bercik, P.; Britz-McKibbin, P. Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics 2019, 15, 82. [Google Scholar] [CrossRef]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, S88–S103. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. [Google Scholar] [CrossRef]
- DiBattista, A.; McIntosh, N.; Lamoureux, M.; Al-Dirbashi, O.Y.; Chakraborty, P.; Britz-Mckibbin, P. Metabolic signatures of cystic fibrosis identified in dried blood spots for newborn screening without carrier identification. J. Proteome Res. 2019, 18, 841–854. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; Van De Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; Van Loon, L.J. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.C.; Rudwill, F.; Breen, L.; Salanova, M.; Blottner, D.; Heise, T.; Heer, M.; Blanc, S.; O’Gorman, D.J. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia 2017, 60, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Stephens, F.B.; Jackman, S.R.; Galera Gordo, J.; Machin, D.J.; Pulsford, R.M.; van Loon, L.J.C.; Wall, B.T. A single day of bed rest, irrespective of energy balance, does not affect skeletal muscle gene expression or insulin sensitivity. Exp. Physiol. 2018, 103, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, S.H.; Hansen, L.S.; Pedersen, M.; Dejgaard, T.; Hansen, J.; Van Hall, G.; Thomsen, C.; Solomon, T.P.J.; Pedersen, B.K.; Krogh-Madsen, R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J. Appl. Physiol. 2012, 113, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Phillips, E.M.; Fielding, R.A. Branched-chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Fabbri, E.; Khadeer, M.A.; Carlson, O.D.; Gonzalez-Freire, M.; Zhang, P.; Semba, R.D.; Ferrucci, L. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef] [PubMed]
- Meynial-Denis, D. Glutamine metabolism in advanced age. Nutr. Rev. 2016, 74, 225–236. [Google Scholar] [CrossRef]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045S–2049S. [Google Scholar] [CrossRef]
- Smith, R.J. Glutamine metabolism and its physiologic importance. J. Parenter. Enter. Nutr. 2009, 14, 40S–44S. [Google Scholar] [CrossRef]
- Mosoni, L.; Patureau Mirand, P.; Houlier, M.; Arnal, M. Age-related changes in protein synthesis measured in vivo in rat liver and gastrocnemius muscle. Mech. Ageing Dev. 1993, 68, 209–220. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Siliprandi, N.; Ciman, M.; Sartorelli, L. Myocardial carnitine transport. Basic Res. Cardiol. 1987, 82, 53–62. [Google Scholar]
- Souza, R.A.; Miranda, H.; Xavier, M.; Lazo-Osorio, R.A.; Gouvea, H.A.; Cogo, J.C.; Vieira, R.P.; Ribeiro, W. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J. Sports Sci. Med. 2009, 8, 672–681. [Google Scholar] [PubMed]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Ward, G.R.; Sale, D.G.; Sutton, J.R. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J. Appl. Physiol. 1977, 43, 700–703. [Google Scholar] [CrossRef]
- Garvey, S.M.; Dugle, J.E.; Kennedy, A.D.; McDunn, J.E.; Kline, W.; Guo, L.; Guttridge, D.C.; Pereira, S.L.; Edens, N.K. Metabolomic profiling reveals severe skeletal muscle group-specific perturbations of metabolism in aged FBN rats. Biogerontology 2014, 15, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Mas, D.; Valdivie, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Rebrin, I.; Sohal, R.S. Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev. 2008, 60, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, M.F.; DiNicolantonio, J.J. An increased need for dietary cysteine in support of glutathione synthesis may underlie the increased risk for mortality associated with low protein intake in the elderly. Age 2015, 37, 96. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.A.; Oliveira, D.; Mansur, H.N.; Fernandes, N.M.; Bastos, M.G. Sarcopenia in chronic kidney disease. J. Bras. Nefrol. 2015, 37, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and clinical impact of organic uremic retention solutes: A comprehensive update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Merlino, A. Gamma-Glutamyl Transpeptidases. VII. In SpringerBriefs in Biochemistry and Molecular Biology; Springer: Basel, Switzerland, 2013; ISBN 978-3-0348-0681-7. [Google Scholar]
- Griffith, O.W.; Bridges, R.J.; Meister, A. Transport of gamma-glutamyl amino acids: Role of glutathione and gamma-glutamyl transpeptidase. Proc. Natl. Acad. Sci. USA 1979, 76, 6319–6322. [Google Scholar] [CrossRef] [PubMed]
- Gotshalk, L.A.; Volek, J.S.; Staron, R.S.; Denegar, C.R.; Hagerman, F.C.; Kraemer, W.J. Creatine supplementation improves muscular performance in older men. Med. Sci. Sports Exerc. 2002, 34, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.; Sue Graves, B.; Cramer, J. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J. Nutr. Health Aging 2007, 11, 459–464. [Google Scholar]
- Gotshalk, L.A.; Kraemer, W.J.; Mendonca, M.A.G.; Vingren, J.L.; Kenny, A.M.; Spiering, B.A.; Hatfield, D.L.; Fragala, M.S.; Volek, J.S. Creatine supplementation improves muscular performance in older women. Eur. J. Appl. Physiol. 2008, 102, 223–231. [Google Scholar] [CrossRef]
- Mignon, M.; Beaufrère, A.M.; Combaret, L.; Meynial-Denis, D. Does long-term intermittent treatment with glutamine improve the well-being of fed and fasted very old rats? J. Parenter. Enter. Nutr. 2007, 31, 456–462. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Hartmane, D.; Lipinska, P.; Wojtowicz, E.; Lysiak-Szydlowska, W.; Olek, R.A. L-carnitine supplementation in older women. A pilot study on aging skeletal muscle mass and function. Nutrients 2018, 10, 255. [Google Scholar] [CrossRef]
- Hassan, B.H.; Hewitt, J.; Keogh, J.W.L.; Bermeo, S.; Duque, G.; Henwood, T.R. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatr. Nurs. 2016, 37, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]





| Baseline (BL) | Step Reduction (SR) | Recovery (RC) | p-Value* | |
|---|---|---|---|---|
| Sex | 10 male, 7 female | - | - | |
| Age (years) | 69 ± 4 | - | - | |
| Body Mass (kg) | 75 ± 14 | 76 ± 15 | 76 ± 15 | 0.26 |
| BMI (kg∙m2) | 27 ± 4 | 27 ± 4 | 27 ± 4 | 0.16 |
| Daily Energy Expenditure (kJ) | 9890 ± 2680 | 8118 ± 1350 | 9498 ± 2310 | 2.99 × 10−4 |
| Myofibrillar Protein Synthesis (% per day) | 1.50 ± 0.06 | 1.33 ± 0.05 | 1.32 ± 0.14 | 0.040 |
| Pedometer Steps (per day) | 7550 ± 3320 | 980 ± 84 | 7345 ± 3850 | 2.70 × 10−6 |
| Armband steps (per day) | 6375 ± 3560 | 1248 ± 850 | 5612 ± 3740 | 1.33 × 10−4 |
| Fasting glucose (mM) | 5.24 ± 0.61 | 5.31 ± 0.92 | 5.47 ± 0.73 | 0.26 |
| (mg/dL) | 94 ± 11 | 96 ± 17 | 99 ± 13 | 0.26 |
| 2h Post-OGTT glucose (mM) | 7.6 ± 1.5 | 9.0 ± 2.4 | 8.2 ± 2.9 | 0.070 |
| (mg/dL) | 137 ± 26 | 163 ± 43 | 147 ± 53 | 0.070 |
| m/z:RMT:Mode | Compound | p-Value | q-Value a | Effect Size b | Pairwise Comparison | BL-SR c | SR-RC c | BL-RC c |
|---|---|---|---|---|---|---|---|---|
| 147.0764:0.908:p | Glutamine | 5.80 × 10−6 | 3.02 × 10−4 | 0.53 | BL-SR, BL-RC | 1.30 | 1.01 | 1.31 |
| Creatine/ Oxoproline * | 1.43 × 10−4 | 1.49 × 10−2 | 0.45 | BL-SR, BL-RC, SR-RC | 2.40 | 0.87 | 2.10 | |
| Glutamine/ Indoxyl Sulfate | 3.01 × 10−4 | 0.015 | 0.40 | BL-SR, BL-RC | 2.65 | 0.90 | 2.39 | |
| Carnitine/ Oxoproline | 1.00 × 10−3 | 0.010 | 0.35 | BL-SR, BL-RC | 1.87 | 0.88 | 1.65 | |
| Creatine/ Indoxyl Sulfate * | 1.42 × 10−3 | 0.015 | 0.42 | BL-SR, BL-RC | 2.70 | 0.82 | 2.21 | |
| Carnitine/ Indoxyl Sulfate | 2.34 × 10−3 | 0.015 | 0.32 | BL-SR, BL-RC | 2.13 | 0.86 | 1.82 | |
| 162.1125:0.666:p | Carnitine (C0) | 2.53 × 10−3 | 0.019 | 0.31 | BL-SR, SR-RC | 1.21 | 0.87 | 1.05 |
| 132.0768:0.710:p | Creatine * | 0.010 | 0.019 | 0.34 | BL-SR, SR-RC | 1.57 | 0.80 | 1.26 |
| 212.0023:1.025:n | Indoxyl sulfate | 0.021 | 0.12 | 0.21 | BL-SR, BL-RC | 0.67 | 1.04 | 0.69 |
| 128.0353:1.025:n | Oxoproline | 0.025 | 0.13 | 0.20 | BL-SR | 0.67 | 1.09 | 0.73 |
| 150.0583:0.890:p | Methionine * | 0.028 | 0.13 | 0.21 | BL-SR | 1.20 | 0.93 | 1.11 |
| 178.051:0.880:n | Hippuric acid | 0.033 | 0.14 | 0.19 | BL-RC | 0.71 | 0.96 | 0.68 |
| 146.1176:0.615:p | Deoxycarnitine * | 0.044 | 0.17 | 0.19 | BL-SR | 1.16 | 0.96 | 1.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saoi, M.; Li, A.; McGlory, C.; Stokes, T.; von Allmen, M.T.; Phillips, S.M.; Britz-McKibbin, P. Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity. Metabolites 2019, 9, 134. https://doi.org/10.3390/metabo9070134
Saoi M, Li A, McGlory C, Stokes T, von Allmen MT, Phillips SM, Britz-McKibbin P. Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity. Metabolites. 2019; 9(7):134. https://doi.org/10.3390/metabo9070134
Chicago/Turabian StyleSaoi, Michelle, Alice Li, Chris McGlory, Tanner Stokes, Mark T. von Allmen, Stuart M. Phillips, and Philip Britz-McKibbin. 2019. "Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity" Metabolites 9, no. 7: 134. https://doi.org/10.3390/metabo9070134
APA StyleSaoi, M., Li, A., McGlory, C., Stokes, T., von Allmen, M. T., Phillips, S. M., & Britz-McKibbin, P. (2019). Metabolic Perturbations from Step Reduction in Older Persons at Risk for Sarcopenia: Plasma Biomarkers of Abrupt Changes in Physical Activity. Metabolites, 9(7), 134. https://doi.org/10.3390/metabo9070134