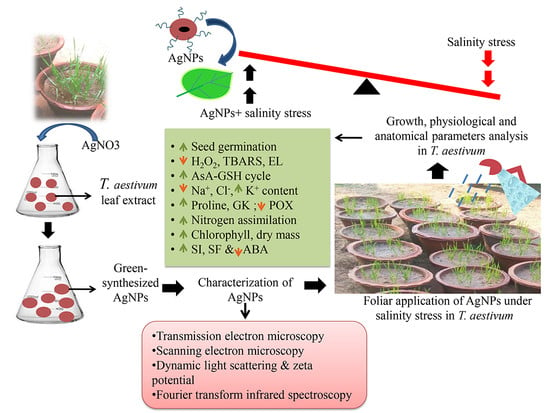
Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wheat Leaves Extract Preparation
2.2. In Vitro Synthesis of Silver Nanoparticles by Wheat
2.3. Characterization of Green Synthesized Silver Nanoparticles
2.4. Plant Material and Growth Conditions
2.5. Estimation of Oxidative Stress Markers
2.6. Estimation of Ions Content
2.7. Estimation of Enzymatic and Non-Enzymatic Antioxidants
2.8. Estimation of Proline Oxidase, Glutamyl Kinase Activity, and Proline Content
2.9. Estimation of Nitrate Reductase, Nitrite Reductase Activities, and Nitrogen Content
2.10. Estimation of Chlorophyll Content and Measurement of Plant Dry Mass
2.11. Measurement of Stomatal Traits
2.12. Estimation of ABA Content
2.13. Estimation of Seed Germination
2.14. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Nanoparticles
3.2. Exogenously Supplied-Silver Nanoparticle Increased Seed Germination Under Salt Stress
3.3. Exogenously Sourced-Sliver Nanoparticles Reduces Oxidative Stress Under Salt Stress
3.4. Exogenously Sourced-Silver Nanoparticles Enhanced Antioxidants Under Salt Stress
3.5. Exogenously Sourced-Sliver Nanoparticle Maintains Na+, K+, and Cl− Content Under Salt Stress
3.6. Exogenously Sourced-Silver Nanoparticles Modulate Proline Metabolism Under Salt Stress
3.7. Exogenously Sourced-Silver Nanoparticles Enhanced Nitrogen Assimilation Under Salt Stress
3.8. Silver Nanoparticle Application Increased Chlorophyll and Plant Dry Mass Under Salt Stress
3.9. Effect of Silver Nanoparticle Application on Stomatal Distribution on the Leaf Epidermis and ABA Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae. 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Romano-Armada, N.; Yanez-Yazlle, M.F.; Irazusta, V.P.; Rajal, V.B.; Moraga, N.B. Potential of bioremediation and PGP traits in Streptomyces as strategies for bio-reclamation of salt-affected soils for agricultural. Pathogens 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Tanveer, M.; Ahmed, H.A.I. ROS signalling in modulating salinity stress tolerance in plants. In Salt and Drought Stress Tolerance in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 299–314. [Google Scholar]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Zelm, E.V.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanism in Plants. Ann. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Banik, N.; Bhattacharjee, S. Complementation of ROS scavenging metabolites with enzymatic antioxidant defense system augments redox-regulation property under salinity stress in rice. Physiol. Mol. Biol. Plants. 2020, 26, 1623–1633. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, D.B.; da Luz, L.M.; de Oliveira, H.O.; Araujo, W.L.; Daloso, D.M.; Fernie, A.R. Metabolomics for understanding stomatal movements. Exp. Plant Physiol. 2019, 31, 9. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticles in autotrophic plants and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Rauwel, P.; Kuunal, S.; Ferdov, S.; Rauwel, E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Effects of nano-materials on seed germination and seedling growth: Striking the slight balance between the concepts and controversies. Mater. Focus. 2016, 5, 195–201. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jin, Q.; Xu, X.; Miao, A.; White, J.C.; Gardea-Torresday, J.L.; Ji, R.; Zhao, L. High-Throughput Screening for Engineered Nanoparticles That Enhances Photosynthesis Using Mesophyll Protoplasts. J. Agric. Food Chem. 2020, 68, 3382–3389. [Google Scholar] [CrossRef]
- Chouhan, N. Silver Nanoparticles: Synthesis, Characterization and Application; IntechOpen: London, UK, 2018; pp. 36–57. [Google Scholar]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mat. Sci. Eng. C. 2020, 27, 110901. [Google Scholar] [CrossRef]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of stevia (Stevia rebaudiana B.) to silver nanoparticles in vitro: Transport and accumulation. Sci. Rep. 2019, 9, 10372. [Google Scholar] [CrossRef] [PubMed]
- Sana, I.; Manaal, Z.; Iram, W.; Abu, B.; Mohammad, R.; Altaf, K.; Naushad, A.; Saheem, A.; Mohd, S.K. Cisplatin bioconjugated enzymatic GNPs amplify the effect of cisplatin with acquiescence. Sci. Rep. 2019, 9, 13826. [Google Scholar]
- Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreases levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Phyiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Hnilickova, H.; Hnilicka, F.; Orsak, M.; Hejnak, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.J.; Smith, S.J.; Miller, A.J. Simultaneously measurement of intracellular pH and K+ or NO3− in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol. 1995, 108, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase: Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop stress and Its Management: Perspectives and Strategies; Springer: Berlin, Germany, 2012; pp. 261–316. [Google Scholar]
- Smith, I.K.; Kendall, A.C.; Keys, A.J.; Turner, J.C.; Lea, P.J. The regulation of the biosynthesis of glutathione in leaves of barley (Hordeumvulgare L.). Plant Sci. 1985, 41, 11–17. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, W.; Yin, L.; Yan, F.; Xu, Y.; Chen, F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron. J. Biotechnol. 2015, 18, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.H.C.; Cavalieri, A.J. Proline oxidase and water stress-induced proline accumulation in spinach leaves. Plant Physiol. 1979, 63, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Hayzer, D.J.; Leisinger, T.H. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. Microbiology. 1980, 118, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mung bean (Vignaradiata, L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kuo, T.-M.; Warner, R.L.; Kleinhofs, A. ln vitro stability of nitrate reductase from barley leaves. Phytochemistry. 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Ramarao, C.S.; Patil, V.H.; Dhak, B.D.; Kadrekar, S.B. A simple in-vivo method for the determination of nitrate reductase activity in rice roots. Z. Pjlanzenphysiol. 1983, 109, 81–85. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Noor, M.A.; Tanveer, M.; Hussain, H.A.; Hussain, S.; Shah, T.; Mehmood, T. Metal toxicity and nitrogen metabolism in plants: An overview. In Carbon and Nitrogen Cycling in Soil; Springer Nature: Singapore, 2020; pp. 221–248. [Google Scholar]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1994, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, E.J. On the causes and ecological significance of stomatal frequency with spatial reference to woodland flora. Philos. Trans. R. Soc. B. 1927, 216, 1–65. [Google Scholar]
- Balasooriya, B.L.W.K.; Samson, R.; Mbikwa, F.; Vitharana, U.W.A.; Boeckx, P.; Van Meirvenne, M. Biomonitoring of urban habitat quality by anatomical and chemical leaf characteristics. Environ. Exp. Bot. 2009, 65, 386–394. [Google Scholar] [CrossRef]
- Hung, K.T.; Kao, C.H. Nitric oxide counteracts the senescence of rice leaves induced by abscisic acid. J. Plant Physiol. 2003, 160, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Ellis, R.H.; Robert, E.H. The Quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Karim, M.A.; Utsunomiya, N.; Shigenaga, S. Effect of sodium chloride on germination and growth of hexaploid triticale at early seedling stage. Japanese, J. Crop Sci. 1992, 61, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Azura, M.S.N.; Zamri, I.; Rashid, M.R.; Shahrin, G.M.; Rafidah, A.R.; Rejab, I.M.; Azima, A.; Suria, M.S.; Amyita, W.U. Evaluation of nanoparticles for promoting seed germination and growth rate in MR263 and MR269 paddy seeds. J. Trop. Agric. Food. Sc. 2017, 45, 13–24. [Google Scholar]
- Almutairi, Z.M. Influence of silver nanoparticles on the salt resistance of tomato (Solanum lycopersicum) during germination. Int. J. Agric. Biol. 2016, 18, 2. [Google Scholar]
- Mohamed, A.K.S.H.; Qayyum, M.F.; Abdel-Haidi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticles on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Abou-Zeid, H.M.; Ismail, G.S.H. The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. to salt stress. Egypt. J. Bot. 2018, 58, 73–85. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D. Arabidopsis K+ efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [Green Version]
- Zaeem, A.; Drouet, S.; Anjum, S.; Khurshid, R.; Younas, M.; Blondeau, J.P.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Effects of Biogenic zinc oxide nanoparticles on growth and oxidative stress response in flax seedlings vs. In Vitro Cultures: A Comparative Analysis. Biomolecules 2020, 10, 918. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Gen. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Ann. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Janicka-Russak, M.; Kabała, K. The role of plasma membrane H+-ATPase in salinity stress of plants. In Progress in Botany; Springer: Cham, Switzerland, 2015; pp. 77–92. [Google Scholar]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signaling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Okuma, E.; Amako, E.; Nakamure, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improve salt tolerance more than glycinebetaine in tobacco bright yellow-2 suspension cultured cells. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Xu, A.; Li, L.; Xie, J.; Wang, X.; Coulter, J.A.; Liu, C.; Wang, L. Effect of long-term nitrogen addition on wheat yield, nitrogen use efficiency, and residual soil nitrate in a semiarid area of the loess plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef] [Green Version]
- Lewis, O.A.M.; Ledi, E.O.; Lips, S.H. Effects of nitrogen source on growth responses to salinity stress in maize and wheat. New Phyotol. 2006, 111, 155–160. [Google Scholar] [CrossRef]
- Bojovoic, B.; Markovic, A. Correlation between Nitrogen and Chlorophyll in Wheat (Triticumaestivum L.). Kragujevac, J. Sci. 2009, 31, 69–74. [Google Scholar]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Queiroz, A.M.; Mezacasa, A.V.; Graciano, D.E.; Falco, W.F.; M’Peko, J.C.; GuimarAfes, F.E.G.; Lawson, T.; Colbeck, J.; Oliveira, S.L.; Caires, A.R.L. Quenching of chlorophyll fluorescence induced by silver nanoparticles. Spectrochem. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Kataria, S.; Jain, M.; Rastogi, A.; Zivcak, M.; Brestic, M.; Liu, S.; Tripathi, D.K. Role of nanoparticles on photosynthesis: Avenues and applications. In Nanomaterials in Plants, Algae, and Microorganisms; Academic Press: Cambridge, MA, USA, 2019; pp. 103–127. [Google Scholar]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orcen, N.; Nazarian, G.; Gharibkhani, M. The responses of stomatal parameters and SPAD value in asian tobacco exposed to chromium. Pol. J. Environ. Stud. 2013, 22, 1441–1447. [Google Scholar]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibole, J.V.; Montero, E.; Cabot, C.; Poschenrieder, C.; Barceló, J. Role of sodium in the ABA-mediated long-term growth response of bean to salt stress. Physiol. Plant. 1998, 104, 299–305. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid –mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Treatments | Seeds Germinated | Germination Rate (%) | Germination Index (%) |
|---|---|---|---|
| Control | 17.67 ± 1.31 b | 73.62 ± 3.16 b | 0.0 ± 0.0 c |
| NaCl | 8.33 ± 1.29 c | 68.04 ± 2.47 c | 92.4 ± 3.24 b |
| AgNP | 29.33 ± 3.93 a | 84.7 ± 4.22 a | 115.1 ± 7.83 a |
| AgNP + NaCl | 18.33 ± 1.69 b | 76.37 ± 3.37 ab | 103.7 ± 4.16 ab |
| Antioxidants | Control | NaCl | AgNP | AgNP + NaCl |
|---|---|---|---|---|
| SOD | 30.33 ± 4.97 b | 59.14 ±6.13 a | 22.14 ± 4.54 b | 33.12 ± 6.72 b |
| APX | 3.2 ± 0.73 c | 4.86 ± 0.93 b | 7.7 ± 0.54 a | 8.86 ± 0.66 a |
| GR | 3.5 ± 0.62 c | 6.44 ± 0.53 b | 10.98 ± 1.68 a | 12.19 ± 1.65 a |
| GPX | 0.026 ± 0.002 d | 0.04 ± 0.002 c | 0.066 ± 0.001 a | 0.048 ± 0.001 b |
| GSH | 320 ± 12.06 c | 365 ± 16.08 bc | 413 ± 19.62 ab | 431 ± 23.77 a |
| AsA | 0.92 ± 0.027 b | 0.74 ± 0.056 c | 1.21 ± 0.027 a | 0.93 ± 0.072 b |
| Treatments | Root | Leaf | ||||
|---|---|---|---|---|---|---|
| Na+ Content | Cl− Content | K+ Content | Na+ Content | Cl− Content | Leaf K+ | |
| Control | 13.4 ± 1.75 b | 15.6 ± 1.94 b | 246 ± 9.44 c | 11.34 ± 1.57 b | 10.12 ± 1.55 b | 226 ± 9.49 b |
| NaCl | 23.03 ± 2.29 a | 25.06 ± 2.64 a | 207 ± 8.18 d | 19.85 ± 3.48 a | 17.56 ± 2.75 a | 167 ± 11.65 c |
| AgNP | 6.7 ± 1.01 c | 4.21 ± 1.11 d | 401 ± 10.71 a | 2.72 ± 1.10 d | 2.13 ± 0.57 c | 346 ± 9.59 a |
| AgNP +NaCl | 6.85 ± 1.84 c | 6.74 ± 1.63 c | 345 ± 8.56 | 4.01 ± 1.38 c | 2.98 ± 0.72 c | 234 ± 12.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahid, I.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems. Biomolecules 2020, 10, 1506. https://doi.org/10.3390/biom10111506
Wahid I, Kumari S, Ahmad R, Hussain SJ, Alamri S, Siddiqui MH, Khan MIR. Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems. Biomolecules. 2020; 10(11):1506. https://doi.org/10.3390/biom10111506
Chicago/Turabian StyleWahid, Iram, Sarika Kumari, Rafiq Ahmad, Sofi J. Hussain, Saud Alamri, Manzer H. Siddiqui, and M. Iqbal R. Khan. 2020. "Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems" Biomolecules 10, no. 11: 1506. https://doi.org/10.3390/biom10111506
APA StyleWahid, I., Kumari, S., Ahmad, R., Hussain, S. J., Alamri, S., Siddiqui, M. H., & Khan, M. I. R. (2020). Silver Nanoparticle Regulates Salt Tolerance in Wheat Through Changes in ABA Concentration, Ion Homeostasis, and Defense Systems. Biomolecules, 10(11), 1506. https://doi.org/10.3390/biom10111506