Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Parasites and Cell Culture
2.3. Parasite Extract
2.4. Synthesis of the SPOT Peptide Array on Cellulose Membrane
2.5. Screening of SPOT Membranes
2.6. Peptide Synthesis and BSA and Biotin Conjugation
2.7. Rabbit Polyclonal Antibodies Production
2.8. Parasite Sample Preparation, SDS-PAGE, and Immunoblotting
2.9. Absorption of Proteins to Pepstatin A-Agarose
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Immunofluorescence Microscopy
2.12. Database Searches, Computational, and Phylogeny Studies
2.13. Ethics Statement
2.14. Statistical Analysis
3. Results
3.1. Spot Synthesis and Epitope Identification
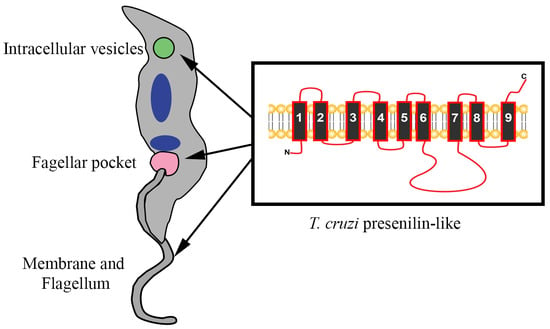
3.2. Structure and Topology of T. cruzi PS-Like
3.3. Motifs Crucial for A22 Aspartyl Protease Family and γ-secretase Activity Are Conserved in T. cruzi Homologs
3.4. Phylogeny and Protein–Protein Interaction
3.5. Antisera Production, SDS-PAGE and Western Blotting
3.6. Subcellular Localization of PS-Like Protein in Different Stages of T. cruzi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Chagas Disease (American Trypanosomiasis) Factsheet; World Health Organization: Geneva, Switzerland, 2015; Available online: http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed on 29 September 2020).
- Silveira, A.; Vinhaes, M. Elimination of vector-borne transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. 1), 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions-A comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, C.; Salazar, C.; Brochero, H.; Teherán, A.; Buitrago, L.S.; Vera, M.; Soto, H.; Florez-Rivadeneira, Z.; Ardila, S.; Parra-Henao, G.; et al. Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: Parasite infection, feeding sources and discrete typing units. Parasit. Vectors 2016, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Vannucchi, V.; Tomberli, B.; Zammarchi, L.; Fornaro, A.; Castelli, G.; Pieralli, F.; Berni, A.; Yacoub, S.; Bartoloni, A.; Olivotto, I. Chagas disease as a cause of heart failure and ventricular arrhythmias in patients long removed from endemic areas: An emerging problem in Europe. J. Cardiovasc. Med. 2014, 16, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.A.; Molina, I.; Murta, S.M.F.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and clinical treatment of Chagas disease: A review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef]
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strain to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife 2018, 7, e34039. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [Green Version]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: The STOP- CHAGAS trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Klemba, M.; Goldberg, D.E. Biological roles of proteases in parasitic protozoa. Annu. Rev. Biochem. 2002, 71, 275–305. [Google Scholar] [CrossRef]
- Maya, J.D.; Cassels, B.K.; Iturriaga-Vasquez, P.; Ferreira, J.; Faundez, M.; Galanti, N.; Ferreira, A.; Morello, A. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 601–620. [Google Scholar] [CrossRef]
- Clayton, J. Chagas disease: Pushing through the pipeline. Nature 2010, 465, S12–S15. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.B. Proteases and protease inhibitors: A balance of activities in host-pathogen interaction. Immunobiology 2006, 211, 263–281. [Google Scholar] [CrossRef] [PubMed]
- McKerrow, J.H.; Caffrey, C.; Kelly, B.; Loke, P.; Sajid, M. Proteases in parasitic diseases. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 497–536. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; De-Simone, S.G.; d’Avila-Levy, C.M.; do Santos, A.L.S.; Nogueira, A.C. Trypanosomatidae peptidases: A target for drugs development. Curr. Enz. Inhib. 2007, 3, 19–48. [Google Scholar] [CrossRef]
- Engel, J.C.; Doyle, P.S.; Hsieh, I.; McKerrow, J.H. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J. Exp. Med. 1998, 188, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKerrow, J.H.; Rosenthal, P.J.; Swenerton, R.; Doyle, P. Development of protease inhibitors for protozoan infections. Curr. Opin. Infect. Dis. 2008, 21, 668–672. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The peptidase database. Nucleic Acids Res. 2010, 38, D227–D233. [Google Scholar] [CrossRef]
- Pinho, R.T.; Beltramini, L.M.; Alves, C.R.; De-Simone, S.G. Trypanosoma cruzi: Isolation and characterization of aspartyl proteases. Exp. Parasitol. 2009, 122, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S. Toward the structure of presenilin/γ-secretase and presenilin homologs. Biochim. Biophys. Acta. 2013, 1828, 2886–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, C.A.; Frykman, S.; Farmery, M.R.; Tjernberg, L.O.; Nilsberth, C.; Pursglove, S.E.; Ito, A.; Winblad, B.; Cowburn, R.F.; Thyberg, J.; et al. Nicastrin, PS, APH-1 and PEN-2 form active γ-secretase complexes in mitochondria. J. Biol. Chem. 2004, 279, 51654–51660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunkan, A.L.; Goate, A.M. PS function and γ-secretase activity. J. Neurochem. 2005, 93, 769–792. [Google Scholar] [CrossRef]
- Sato, T.; Diehl, T.S.; Narayanan, S.; Funamoto, S.; Ihara, Y.; De Strooper, B.; Steiner, H.; Haass, C.; Wolfe, M.S. Active γ-secretase complexes contain only one of each component. J. Biol. Chem. 2007, 282, 33985–33993. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, L.G.; De Castro-Borges, W.; De Souza Gomes, M.; Guerra-Sá, R.; Rodrigues, V. Molecular cloning, sequencing and expression analysis of PS cDNA from Schistosoma mansoni. Parasitol. Res. 2009, 106, 7–13. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Oh, S.S.; Chishti, A.H. A PS-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion. Mol. Biochem. Parasitol. 2008, 158, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Singh, V.; Singh, S. Structural insights into key plasmodium proteases as therapeutic drug targets. Front. Microbiol. 2019, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Henricson, A.; Sonnhammer, E.L.L.; Baillie, D.L.; Gomes, A.V. Functional characterization in Caenorhabditis elegans of transmembrane worm-human orthologs. BMC Genom. 2004, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Giovanni-De-Simone, S.; De Carvalho, L.C.P.; Oliva, O.F.P.; Andrade, S.G.; Galvão-Castro, B. Trypanosoma cruzi strain-specific monoclonal antibodies: Identification of Colombian strain flagellates in the insect vector. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 750–754. [Google Scholar] [CrossRef]
- Piras, M.M.; Piras, R.; Henriquez, D. Changes in morphology and infectivity of cell culture-derived trypomastigotes of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1982, 6, 67–81. [Google Scholar] [CrossRef]
- Frank, R. The SPOT-synthesis technique. Synthetic peptide arrays on membrane support-principles and applications. J. Immunol. Methods 2002, 267, 13–26. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; De-Simone, T.S. Spot Synthesis: An optimized microarray to detect IgE epitopes. Methods Mol. Biol. 2016, 1352, 263–277. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Teixeira-Pinto, L.A.L.; Santos, J.D.L.; De-Simone, T.S.; Melgarejo, A.R.; Aguiar, A.S.; Marchi-Salvador, D.P. Linear B-cell epitopes in BthTX-1, BthTX-II and BthA-1, phospholipase A2’s from Bothrops jararacussu snake venom, recognized by therapeutically neutralizing commercial horse antivenom. Toxicon 2013, 72, 90–101. [Google Scholar] [CrossRef]
- Eid, M.; Evin, G.; Castro, B.; Menard, J.; Corvol, P. New renin inhibitors are homologous with pepstatin. Biochem. J. 1981, 197, 465–471. [Google Scholar] [CrossRef] [Green Version]
- De-Simone, S.G.; Nascimento, H.J.; Prado, I.; Aguiar, A.S.; Melgarejo, A.R.; Pina, J.L.; Ferreira, P.F.; Provance, D.W., Jr. Purification of equine IgG3 by lectin affinity and an interaction analysis via microscale thermophoresis. Anal. Biochem. 2018, 561–562, 27–31. [Google Scholar] [CrossRef]
- Demmel, L.; Schmidt, K.; Lucast, L.; Havlicek, K.; Zankel, A.; Koestler, T.; Reithofer, V.; de Camilli, P.; Warren, G. The endocytic activity of the flagellar pocket in Trypanosoma brucei is regulated by an adjacent phosphatidylinositol phosphate kinase. J. Cell. Sci. 2014, 127, 2351–2364. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, K.; Stoffel, W. TMbase-A database of membrane-spanning protein segments. Biol. Chem. Hoppe Seyler 1993, 347, 166. [Google Scholar]
- Bernse, A.; Viklund, H.; Hennerdal, A.; Elofsson, A. TOPCONS: Consensus prediction of membrane protein topology. Nucleic Acids Res. 2009, 37, W465–W468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hönigschmid, P.; Frishman, D. Accurate prediction of helix interactions and residue contacts in membrane proteins. J. Struct. Biol. 2016, 194, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.R.; Yu, G. Assembly, maturation and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in PS-1 required for PS endoproteolysis and γ-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Xia, W.; Rahmati, T.; Wolfe, M.S.; Selkoe, D.J. The transmembrane aspartates in PS 1 and 2 are obligatory for γ-secretase activity and amyloid β-protein generation. J. Biol. Chem. 2000, 275, 3173–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Sato, K.; Liou, W.; Pant, S.; Harada, A.; Grant, B.D. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008, 27, 1183–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, A. The GxGD Motif of PS contributes to catalytic function and substrate identification of γ-Secretase. J. Neurosci. 2006, 26, 3821–3828. [Google Scholar] [CrossRef]
- Berná, L.; Chiribao, M.L.; Greif, G.; Rodriguez, M.; Alvarez-Valin, F.; Robello, C. Transcriptomic analysis reveals metabolic switches and surface remodeling as key processes for stage transition in Trypanosoma cruzi. PeerJ 2017, 5, e3017. [Google Scholar] [CrossRef] [Green Version]
- Kuo, I.Y.; Hu, J.; Ha, Y.; Ehrlich, B.E. PS-like GxGD membrane proteases have dual roles as proteolytic enzymes and ion channels. J. Biol. Chem. 2015, 290, 6419–6427. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Pettingell, W.H.; Jung, Y.K.; Kovacs, D.M.; Tanzi, R.E. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 1997, 18, 373–376. [Google Scholar] [CrossRef]
- Walter, J.; Grünberg, J.; Capell, A.; Pesold, B.; Schindzielorz, A.; Citron, M.; Mendla, K.; St George-Hyslop, P.; Multhaup, G.; Selkoe, D.J.; et al. Proteolytic processing of the Alzheimer disease-associated presenilin-1 generates an in vivo substrate for protein kinase C. Proc. Natl. Acad. Sci. USA 1997, 94, 5349–5354. [Google Scholar] [CrossRef] [Green Version]
- Evin, G.; Sharples, R.A.; Weidemann, A.; Reinhard, F.B.; Carbone, V.; Culvenor, J.G.; Holsinger, R.M.; Sernee, M.F.; Beyreuther, K.; Masters, C.L. Aspartyl protease inhibitor pepstatin binds to the presenilins of Alzheimer’s disease. Biochemistry 2001, 40, 8359–8368. [Google Scholar] [CrossRef] [PubMed]
- Elad, N.; De Strooper, B.; Lismont, S.; Hagen, W.; Veugelen, S.; Arimon, M.; Horré, K.; Berezovska, O.; Sachse, C.; Chávez-Gutiérrez, L. The dynamic conformational landscape of gamma-secretase. J. Cell. Sci. 2015, 128, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roderick, J.E.; Tesell, J.; Shultz, L.D.; Brehm, M.A.; Greiner, D.; Harris, M.H.; Silverman, L.B.; Sallan, S.E.; Gutierrez, A.; Look, A.T.; et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood 2014, 123, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morohashi, Y.; Kan, T.; Tominari, Y.; Fuwa, H.; Okamura, Y.; Watanabe, N.; Sato, C.; Natsugari, H.; Fukuyama, T.; Iwatsubo, T.; et al. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenyl glycine t-butyl ester). J. Biol. Chem. 2006, 281, 14670–14676. [Google Scholar] [CrossRef] [Green Version]
- Sogorb-Esteve, A.; García-Ayllón, M.S.; Llansola, M.; Felipo, V.; Blennow, K.; Sáez-Valero, J. Inhibition of γ-secretasel leads to an increase in presenilin-1. Mol. Neurobiol. 2018, 55, 5047–5058. [Google Scholar] [CrossRef] [Green Version]
- Barthet, G.; Shioi, J.; Shao, Z.; Ren, Y.; Georgakopoulos, A.; Robakis, N.K. Inhibitors of γ-secretase stabilize the complex and differentially affect processing of amyloid precursor protein and other substrates. FASEB J. 2011, 25, 2937–2946. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Neely, K.M.; Green, K.N.; LaFerla, F.M. Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a γ-secretase-independent manner. J. Neurosci. 2011, 31, 2781–2791. [Google Scholar] [CrossRef] [Green Version]
- Gama Sosa, M.A.; De Gasperi, R.; Hof, P.R.; Elder, G.A. Fibroblast growth factor rescues brain endothelial cells lacking presenilin 1 from apoptotic cell death following serum starvation. Sci. Rep. 2016, 6, 30267. [Google Scholar] [CrossRef] [Green Version]
- Durante, I.M.; Cámara, M.M.; Buscaglia, C.A. A novel Trypanosoma cruzi protein associated to the flagellar pocket of replicative stages and involved in parasite growth. PLoS. ONE 2015, 10, e0130099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, T.; De Strooper, B. Presenilins: Members of the gamma-secretase quartets, but part-time soloists too. Physiology 2008, 23, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Ver. Microbiol. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- McConville, M.J.; Mullin, K.A.; Ilgoutz, S.C.; Teasdale, R.D. Secretory pathway of Trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 2003, 66, 122–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Haapasalo, A.; Doo, Y.K.; MacKenzie Ingano, L.A.; Pettingell, W.H.; Kovacs, D.M. PS/γ-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J. 2006, 20, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Kessler, R.L.; Eger, I.; Soares, M.J. Trypanosoma cruzi intracellular amastigotes isolated by nitrogen decompression are capable of endocytosis and cargo storage in reservosomes. PLoS ONE 2015, 10, e0130165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfim-Melo, A.; Ferreira, E.R.; Florentino, P.; Mortara, R.A. Amastigote synapse: The tricks of Trypanosoma cruzi extracellular amastigotes. Front. Microbiol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- White, R.E.; David, J.; Powell, D.J.; Berry, C. HIV proteinase inhibitors target the Ddi1-like protein of Leishmania parasites. FASEB J. 2016, 25, 1729–1736. [Google Scholar] [CrossRef]
- Sibley, L.D. The roles of intramembrane proteases in protozoan parasites. Biochim. Biophys. Acta. 2013, 1828, 2908–2915. [Google Scholar] [CrossRef] [Green Version]
- Conte, I.; Labriola, C.; Cazzulo, J.J.; Docampo, R.; Parodi, A.J. The interplay between folding-facilitating mechanisms in Trypanosoma cruzi endoplasmic reticulum. Mol. Biol. Cell 2003, 14, 3529–3540. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Docampo, R. Membrane proteins in Trypanosomatids involved in Ca2+ homeostasis and signaling. Genes 2018, 9, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; McBrayer, M.K.; Wolfe, D.M.; Haslett, L.J.; Kumar, A.; Sato, Y.; Lie, P.P.; Mohan, P.; Coffey, E.E.; Kompella, U.; et al. Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell. Rep. 2015, 12, 1430–1444. [Google Scholar] [CrossRef] [Green Version]
- Guyett, P.J.; Xia, S.; Swinney, D.C.; Pollastri, M.P.; Mensa-Wilmot, K. Glycogen synthase kinase 3β promotes the endocytosis of transferrin in the African Trypanosome. ACS Infect. Dis. 2016, 2, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; LaVoie, M.J.; Ostaszewski, B.L.; Ye, W.; Wolfe, M.S.; Selkoe, D.J. Gama-secretase is a membrane protein complex comprised of PS, nicastrin, Aph-1 and Pen-2. Proc. Natl. Acad. Sci. USA 2003, 100, 6382–6387. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, A.; Tani, K.; Yamamoto, A.; Kitamura, N.; Komada, M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol. Biol. Cell 2006, 17, 4876–4887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Beher, D.; Nyborg, A.C.; Shearman, M.S.; Golde, T.E.; Goate, A. C-terminal PAL motif of PS and PS homologs required for normal active site conformation. J. Neurochem. 2006, 96, 218–227. [Google Scholar] [CrossRef]
- Marinangeli, C.; Tasiaux, B.; Opsomer, R.; Hage, S.; Sodero, A.O.; Dewachter, I.; Octave, J.N.; Smith, S.O.; Constantinescu, S.N.; Kienlen-Campard, P. PS transmembrane domain 8 conserved AXXXAXXXG motifs are required for the activity of the γ-secretase complex. J. Biol. Chem. 2015, 290, 7169–7184. [Google Scholar] [CrossRef] [Green Version]





| Epitope Code | Epitope Sequence | Residue Position |
|---|---|---|
| EP1 | AFLLGRRIA | 11–19 |
| EP2 | SLIADQQFS | 41–49 |
| EP3 | IFSFMDEEA | 51–58 |
| EP4 | ALYDMVAVLSPRGP | 199–204 |
| EP5 | KRNEPL | 214–219 |
| EP6 | YNSNANPSMQKA | 224–235 |
| EP7 | PPGEDMHTRDGPRE | 241–254 |
| EP8 | SVPRLYYA | 271–278 |
| EP9 | RSPFKLGLGD | 281–290 |
| EP10 | KSRF | 331–334 |
| EP11 | RFVVT | 355–359 |
| TMD | T. cruzi PS-Like | aa Position * | Human PS | AA Position * |
|---|---|---|---|---|
| 1st | WSVLN | 34–38 | ATIKS | 98–102 |
| 2nd | SIVNALILVA | 70–79 | SILNAAIMIS | 132–141 |
| 2nd | MV | 88–89 | MV | 93–94 |
| 5th | SVIVG | 168–172 | SALMA | 230–234 |
| 6th | YD | 193–194 | YD | 256–257 |
| 6th | MV | 195–196 | MV | 298–299 |
| - | NSSND | 256–260 | AQRDS | 342–346 |
| - | - | - | SSILA | 366–370 |
| 7th | PFKLGLGD | 283–290 | GVKLGLGD | 378–385 |
| 7th | SVLSARAAL | 295–303 | SVLVGKASA | 390–398 |
| 8th | ASTVAVCFG | 312–320 | ACFVAILIG | 409–417 |
| 9th | PALP | 339–342 | PALP | 433–436 |
| 9th | ALPALPISICFG | 336–347 | ALPALPISITFG | 431–442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechuga, G.C.; Napoleão-Pêgo, P.; Bottino, C.C.G.; Pinho, R.T.; Provance-Jr, D.W.; De-Simone, S.G. Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization. Biomolecules 2020, 10, 1564. https://doi.org/10.3390/biom10111564
Lechuga GC, Napoleão-Pêgo P, Bottino CCG, Pinho RT, Provance-Jr DW, De-Simone SG. Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization. Biomolecules. 2020; 10(11):1564. https://doi.org/10.3390/biom10111564
Chicago/Turabian StyleLechuga, Guilherme C., Paloma Napoleão-Pêgo, Carolina C. G. Bottino, Rosa T. Pinho, David W. Provance-Jr, and Salvatore G. De-Simone. 2020. "Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization" Biomolecules 10, no. 11: 1564. https://doi.org/10.3390/biom10111564
APA StyleLechuga, G. C., Napoleão-Pêgo, P., Bottino, C. C. G., Pinho, R. T., Provance-Jr, D. W., & De-Simone, S. G. (2020). Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization. Biomolecules, 10(11), 1564. https://doi.org/10.3390/biom10111564