Tautomerism of Guanine Analogues
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
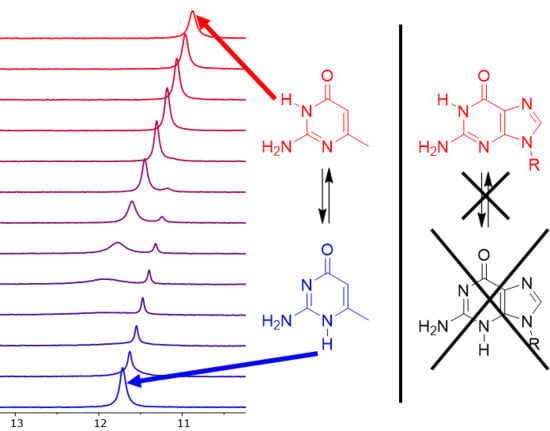
3.1. Computation—Monomers
3.2. Experiments
3.3. Computations—Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids—A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D.; Crick, F.H.C. Genetical Implications of the Structure of Deoxyribonucleic Acid. Nature 1953, 171, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Löwdin, P.O. Proton Tunneling in DNA and Its Biological Implications. Rev. Mod. Phys. 1963, 35, 724–732. [Google Scholar] [CrossRef]
- Pérez, A.; Tuckerman, M.E.; Hjalmarson, H.P.; von Lilienfeld, O.A. Enol Tautomers of Watson-Crick Base Pair Models Are Metastable Because of Nuclear Quantum Effects. J. Am. Chem. Soc. 2010, 132, 11510–11515. [Google Scholar] [CrossRef] [PubMed]
- Florián, J.; Leszczyński, J. Spontaneous DNA mutations induced by proton transfer in the guanine cytosine base pairs: An energetic perspective. J. Am. Chem. Soc. 1996, 118, 3010–3017. [Google Scholar] [CrossRef]
- Singh, V.; Fedeles, B.; Essigmann, J.M. Role of tautomerism in RNA biochemistry. RNA 2017, 21, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, J.C.; Strobel, S.A. Catalytic strategies of self-cleaving ribozymes. Acc. Chem. Res. 2008, 41, 1027–1035. [Google Scholar] [CrossRef]
- Hanus, M.; Ryjáček, F.; Kabeláč, M.; Kubar, T.; Bogdan, T.V.; Trygubenko, S.A.; Hobza, P. Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Guanine: Surprising stabilization of rare tautomers in aqueous solution. J. Am. Chem. Soc. 2003, 125, 7678–7688. [Google Scholar] [CrossRef]
- Dolgounitcheva, O.; Zakrzewski, V.G.; Ortiz, J.V. Electron propagator theory of guanine and its cations: Tautomerism and photoelectron spectra. J. Am. Chem. Soc. 2000, 122, 12304–12309. [Google Scholar] [CrossRef]
- Colominas, C.; Luque, F.J.; Orozco, M. Tautomerism and protonation of guanine and cytosine. Implications in the formation of hydrogen-bonded complexes. J. Am. Chem. Soc. 1996, 118, 6811–6821. [Google Scholar] [CrossRef]
- Stasyuk, O.A.; Szatylowicz, H.; Krygowski, T.M. Effect of the H-Bonding on Aromaticity of Purine Tautomers. J. Org. Chem. 2012, 77, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. The influence of intermolecular halogen bonds on the tautomerism of nucleobases. I. Guanine. Tetrahedron 2015, 71, 5260–5266. [Google Scholar] [CrossRef]
- Hobza, P.; Šponer, J. Structure, energetics, and dynamics of the nucleic acid base pairs: Nonempirical ab initio calculations. Chem. Rev. 1999, 99, 3247–3276. [Google Scholar] [CrossRef] [PubMed]
- Piuzzi, F.; Mons, M.; Dimicoli, I.; Tardivel, B.; Zhao, Q. Ultraviolet spectroscopy and tautomerism of the DNA base guanine and its hydrate formed in a supersonic jet. Chem. Phys. 2001, 270, 205–214. [Google Scholar] [CrossRef]
- Mons, M.; Dimicoli, I.; Piuzzi, F.; Tardivel, B.; Elhanine, M. Tautomerism of the DNA base guanine and its methylated derivatives as studied by gas-phase infrared and ultraviolet spectroscopy. J. Phys. Chem. A 2002, 106, 5088–5094. [Google Scholar] [CrossRef]
- Li, W.; Jin, J.; Liu, X.Q.; Wang, L. Structural Transformation of Guanine Coordination Motifs in Water Induced by Metal Ions and Temperature. Langmuir 2018, 34, 8092–8098. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, L.; Ding, Y.Q.; Sun, Q.; Xu, W. Real-Space Evidence of Rare Guanine Tautomer Induced by Water. ACS Nano 2016, 10, 3776–3782. [Google Scholar] [CrossRef]
- Andersen, E.S.; Dong, M.D.; Nielsen, M.M.; Jahn, K.; Lind-Thomsen, A.; Mamdouh, W.; Gothelf, K.V.; Besenbacher, F.; Kjems, J. DNA origami design of dolphin-shaped structures with flexible tails. ACS Nano 2008, 2, 1213–1218. [Google Scholar] [CrossRef]
- Xu, W.; Kelly, R.E.A.; Gersen, H.; Laegsgaard, E.; Stensgaard, I.; Kantorovich, L.N.; Besenbacher, F. Prochiral Guanine Adsorption on Au(111): An Entropy-Stabilized Intermixed Guanine-Quartet Chiral Structure. Small 2009, 5, 1952–1956. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Portalone, G.; Colapietro, M. Redetermination of isocytosine. Acta Crystallogr. E 2007, 63, O1869–O1871. [Google Scholar] [CrossRef]
- Dračínský, M.; Jansa, P.; Ahonen, K.; Buděšínský, M. Tautomerism and the Protonation/Deprotonation of Isocytosine in Liquid- and Solid-States Studied by NMR Spectroscopy and Theoretical Calculations. Eur. J. Org. Chem. 2011, 2011, 1544–1551. [Google Scholar] [CrossRef]
- Dračínský, M.; Hodgkinson, P. Solid-state NMR studies of nucleic acid components. RSC Adv. 2015, 5, 12300–12310. [Google Scholar] [CrossRef] [Green Version]
- Pohl, R.; Socha, O.; Šála, M.; Rejman, D.; Dračínský, M. The Control of the Tautomeric Equilibrium of Isocytosine by Intermolecular Interactions. Eur. J. Org. Chem. 2018, 2018, 5128–5135. [Google Scholar] [CrossRef]
- Pohl, R.; Socha, O.; Slavíček, P.; Šála, M.; Hodgkinson, P.; Dračínský, M. Proton transfer in guanine-cytosine base pair analogues studied by NMR spectroscopy and PIMD simulations. Faraday Discuss. 2018, 212, 331–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dračínský, M.; Jansa, P.; Chocholoušová, J.; Vacek, J.; Kovačková, S.; Holý, A. Mechanism of the Isotopic Exchange Reaction of the 5-H Hydrogen of Uracil Derivatives in Water and Nonprotic Solvents. Eur. J. Org. Chem. 2011, 2011, 777–785. [Google Scholar] [CrossRef]
- Štoček, J.R.; Bártová, K.; Čechová, L.; Šála, M.; Socha, O.; Janeba, Z.; Dračínský, M. Determination of nucleobase-pairing free energies from rotamer equilibria of 2-(methylamino)pyrimidines. Chem. Commun. 2019, 55, 11075–11078. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry 3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Gerhardt, V.; Tutughamiarso, M.; Bolte, M. Pseudopolymorphs of 2,6-diaminopyrimidin-4-one and 2-amino-6-methylpyrimidin-4-one: One or two tautomers present in the same crystal. Acta Crystallogr C 2011, 67, O179–O187. [Google Scholar] [CrossRef] [PubMed]





| R5 | R6 | 2,3-I (keto) | 1,2-I (keto) | 2,4-I (enol) | 1,3-I (imino) 1 | |
|---|---|---|---|---|---|---|
| 1 | H | H | 0.0 | 13.9 | 20.7 | 23.3 |
| 2 | CH3 | H | 0.0 | 12.0 | 30.5 | 23.8 |
| 3 | t-butyl | H | 0.0 | 12.8 | 22.0 | 22.3 |
| 4 | NH2 | H | 0.0 | 6.5 | 25.9 | 29.6 |
| 5 | CF3 | H | 0.0 | 16.3 | 24.3 | 28.7 |
| 6 | NO2 | H | 0.0 | 24.9 | 24.6 | 34.8 |
| 7 | H | CH3 | 0.0 | 12.6 | 28.2 | 21.3 |
| 8 | H | t-butyl | 0.0 | 12.8 | 27.8 | 22.3 |
| 9 | H | NH2 | 0.0 | 34.8 | 28.0 | 36.7 |
| 10 | H | CF3 | 0.0 | 32.3 | 29.0 | 45.3 |
| 11 | H | NO2 | 0.0 | 32.5 | 20.0 | 49.1 |
| 2,3-I (keto) | 1,2-I (keto) | 2,4-I (enol) | 1,3-I (imino) 1 | |
|---|---|---|---|---|
| 12 | 0.0 | 41.0 | 33.8 | 49.0 |
| 13 | 0.0 | 17.2 | 34.3 | 32.6 |
| 14 | 0.0 | 38.5 | 38.3 | 45.1 |
| 15 | 0.0 | 11.5 | 48.7 | 27.3 |
| 16 | 0.0 | 8.5 | 46.8 | 20.1 |
| 17 | 0.0 | 10.8 | 30.6 | 21.0 |
| Dimer/Complex Structure | Ecomplex | Estabil |
|---|---|---|
| 1(2,3-I) + 1(1,2-I) | −79.9 | −66.0 |
| 7(2,3-I) + 7(1,2-I) | −80.1 | −67.5 |
| 16(2,3-I) + 16(1,2-I) | −77.3 | −68.8 |
| 10(2,3-I) + 10(1,2-I) | −79.5 | −47.2 |
| 12(2,3-G) + 12(1,2-G) | −78.1 | −37.2 |
| 7(1,2-I) + 12(2,3-G) | −79.2 | −66.6 |
| 16(1,2-I) + 12(2,3-G) | −78.1 | −69.7 |
| 1(2,4-I) + T | −68.7 | −47.9 |
| 1(1,3-I) + DAP | −55.0 | −32.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štoček, J.R.; Dračínský, M. Tautomerism of Guanine Analogues. Biomolecules 2020, 10, 170. https://doi.org/10.3390/biom10020170
Štoček JR, Dračínský M. Tautomerism of Guanine Analogues. Biomolecules. 2020; 10(2):170. https://doi.org/10.3390/biom10020170
Chicago/Turabian StyleŠtoček, Jakub Radek, and Martin Dračínský. 2020. "Tautomerism of Guanine Analogues" Biomolecules 10, no. 2: 170. https://doi.org/10.3390/biom10020170
APA StyleŠtoček, J. R., & Dračínský, M. (2020). Tautomerism of Guanine Analogues. Biomolecules, 10(2), 170. https://doi.org/10.3390/biom10020170