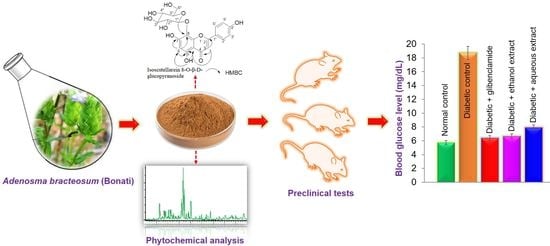
Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Plant Extracts and Isolation
2.3. In Vivo Confirmatory Studies
2.3.1. Experimental Animals
2.3.2. Preliminary Phytochemical Investigation
2.3.3. Determination of Total Phenolic Content
2.3.4. Determination of Total Flavonoid Content
2.3.5. Free Radical Scavenging Activity Assay
2.3.6. ABTS Radical Scavenging Assay
2.3.7. In Vitro α-glucosidase Inhibition Assay
2.3.8. Toxicity Determination of Extracts
2.3.9. Evaluation of Extracts in Streptozotocin-Induced Diabetic Mice
2.3.10. Anti-Hyperglycemic Assay on Glucose Loaded Mice
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Total Flavonoid Content
3.2. Chemical Structure Determination of the Separated Compound
3.3. In Vitro α-Glucosidase Inhibition Assay
3.4. Effect of Plant Extracts on Blood Glucose Levels in STZ-Induced Diabetes in Mice
3.5. Free Radical Scavenging Determined by DPPH and ABTS Assays Of Extracts and IG Compound from A. bracteosum
3.6. In Vitro α-Glucosidase Inhibition of IG
3.7. Oral Glucose Tolerance Tests for the Eevaluation of the Anti-Hyperglycemic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Magliano, D.J.; Islam, R.M.; Barr, E.L.M.; Gregg, E.W.; E Pavkov, M.; Harding, J.L.; Tabesh, M.; Koye, D.N.; E Shaw, J. Trends in incidence of total or type 2 diabetes: Systematic review. BMJ 2019, 366, l5003. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Nogueira-Machado, J.A.; Chaves, M.M. From hyperglycemia to AGE-RAGE interaction on the cell surface: A dangerous metabolic route for diabetic patients. Expert Opin. Ther. Targets 2008, 12, 871–882. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiba, T.; Inoguchi, T.; Sportsman, J.R.; Heath, W.F.; Bursell, S.; King, G.L. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am. J. Physiol. Metab. 1993, 265, E783–E793. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; An, S.S.A.; Hulme, J.P. Mitochondrial therapeutic interventions in Alzheimer’s disease. J. Neurol. Sci. 2018, 395, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sabu, M.C.; Kuttan, R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002, 81, 155–160. [Google Scholar] [CrossRef]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 105–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungurianu, A.; Şeremet, O.; Gagniuc, E.; Olaru, O.T.; Guţu, C.; Grǎdinaru, D.; Ionescu-Tȋrgovişte, C.; Marginǎ, D.; Dǎnciulescu-Miulescu, R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019, 150, 104522. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Rashidi, A.A.; Taherian, A.A; Vakili, Z.; Sajad Sajadian, M.; Ghardashi, M. Antidiabetic and Antihyperlipidemic Effects of Ethanol Extract of Rosa canina L. fruit on Diabetic Rats: An Experimental Study With Histopathological Evaluations. J. Evid.-Based Complementary Altern. Med. 2016, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Wang, J.; Pang, X.; Zhuang, X.; Zhu, X.; Qu, W. Hypoglycemic effect of aqueous extract of seabuckthorn (Hippophae rhamnoides L.) seed residues in streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, 228–232. [Google Scholar] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; De Luca, D.; De Cindio, B.; Menichini, F. Comparative Study on the Chemical Composition, Antioxidant Properties and Hypoglycaemic Activities of Two Capsicum annuum L. Cultivars (Acuminatum small and Cerasiferum). Plant Foods Hum. Nutr. 2011, 66, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, E.T.; Kuleva, L.V.; Thanh, L.T. Composition of the Essential Oil of Adenosma bracteosum Bonati. J. Essent. Oil Res. 1994, 6, 305–306. [Google Scholar] [CrossRef]
- Hong, N.N.; Han, L.T.N.; Thang, P.Q. Antioxidant activity and anti-hyperglycemic effect from Adenosma bracteosum Bonati. Tạp chí Sinh học 2018, 40. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of totalphenolics with phospho-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.D.C.; Pereira, Z.V. Evaluation of Antioxidant Activity, Total Flavonoids, Tannins and Phenolic Compounds in Psychotria Leaf Extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Der Duh, P. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Li, M.; Zhu, F.; Liu, F.-L.; Huang, J.-B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Sadasivan, S.; Latha, P.; Sasikumar, J.; Rajashekaran, S.; Shyamal, S.; Shine, V. Hepatoprotective studies on Hedyotis corymbosa (L.) Lam. J. Ethnopharmacol. 2006, 106, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kifayatullah, M.; Mustafa, M.S.; Sengupta, P.; Sarker, M.R.; Das, A.; Das, S.K.; Senguptha, P. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J. Acute Dis. 2015, 4, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kojima, R.; Ito, M. Strain differences in the diabetogenic activity of streptozotocin in mice. Boil. Pharm. Bull. 2006, 29, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Joy, K.; Kuttan, R. Anti-diabetic activity of Picrorrhiza kurroa extract. J. Ethnopharmacol. 1999, 67, 143–148. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.; Malleshi, N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. New York Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetol. 2008, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative Stress and Diabetic Vascular Complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. 3rd Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giau, V.V.; Bagyinszky, E.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic Factors of Cerebral Small Vessel Disease and Their Potential Clinical Outcome. Int. J. Mol. Sci. 2019, 20, 4298. [Google Scholar] [CrossRef] [Green Version]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A. Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 4149. [Google Scholar] [CrossRef] [Green Version]
- Giau, V.V.; Lee, H.; An, S.S.A; Hulme, J. Recent advances in the treatment of C. difficile using biotherapeutic agents. Infect. Drug Resist. 2019, 12, 1597–1615. [Google Scholar] [CrossRef] [Green Version]
- Van Giau, V.; An, S.S.A.; Hulme, J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des. Devel. Ther. 2019, 13, 327–343. [Google Scholar] [CrossRef] [Green Version]



| Samples | Total Phenolic Content (mg GAE/g extract) | Total Flavonoid Content (mg RE/g extract) |
|---|---|---|
| Ethanol extract | 241.47 ± 4.47 a | 101.99 ± 4.48 a |
| Aqueous extract | 225.91 ± 2.54 b | 26.13 ± 0.76 b |
| Samples | DPPH Assay IC50 (μg/mL) | ABTS Assay IC50 (μg/mL) |
|---|---|---|
| Ethanol extract | 6.61 ± 0.17 b | 10.33 ± 0.10 b |
| Aqueous extract | 10.12 ± 0.13 c | 11.17 ± 0.05 c |
| IG compound | 11.93 ± 1.23 d | 11.41 ± 0.63 c |
| Standard (ascorbic acid) | 2.89 ± 0.09 a | 5.25 ± 0.62 a |
| Samples | α-Glucosidase Inhibition IC50 (μg/mL) |
|---|---|
| Ethanol extract | 26.55 ± 1.57 b |
| Aqueous extract | 42.12 ± 3.02 c |
| IG compound | 1.40 ± 0.19 a |
| Acarbose | 87.94 ± 4.08 d |
| Treatment | Dose | Blood Glucose (mmol/L) | % Inhibition (Compared with Group II) |
|---|---|---|---|
| Normal group (blank control) | Not observed | 6.42 ± 0.47 cd | Not observed |
| Group I (Glucose) | 2 g/kg | 12.05 ± 0.62 a | Not observed |
| Group II (Glibenclamide) | 10 mg/kg | 5.91 ± 0.38 d | 56.68 |
| Group III (aqueous extract) | 40 mg/kg | 7.66 ± 0.29 b | 36.43 |
| Group IV (aqueous extract) | 50 mg/kg | 5.88 ± 1.26 d | 51.20 |
| Group V (ethanol extract) | 40 mg/kg | 6.92 ± 0.38 c | 42,57 |
| Group VI (ethanol extract) | 50 mg/kg | 5.48 ± 0.40 d | 54.52 |
| Group VII (IG) | 10 mg/kg | 6.35 ± 0.11cd | 47.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.H.; Pham, Q.T.; Luong, T.N.H.; Le, H.K.; Vo, V.G. Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules 2020, 10, 201. https://doi.org/10.3390/biom10020201
Nguyen NH, Pham QT, Luong TNH, Le HK, Vo VG. Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules. 2020; 10(2):201. https://doi.org/10.3390/biom10020201
Chicago/Turabian StyleNguyen, Ngoc Hong, Quang Thang Pham, Thi Ngoc Han Luong, Hoang Khai Le, and Van Giau Vo. 2020. "Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati)" Biomolecules 10, no. 2: 201. https://doi.org/10.3390/biom10020201
APA StyleNguyen, N. H., Pham, Q. T., Luong, T. N. H., Le, H. K., & Vo, V. G. (2020). Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules, 10(2), 201. https://doi.org/10.3390/biom10020201