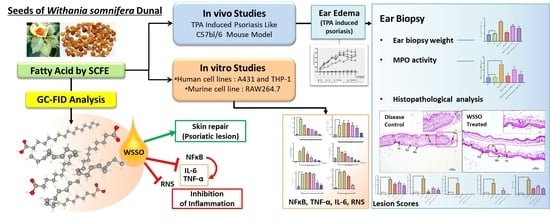
Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Isolation of WSSO from W. somnifera Seeds
2.3. Measurement of Fatty Acid Contents in WSSO
2.4. HPLC Analysis of WSSO, and Post SCFE Exhaust of WS Seeds
2.5. In Vivo Studies for Anti-Inflammatory and Anti-Psoriasis Potential
2.5.1. Experimental Animals
2.5.2. Generation of TPA-Induced Psoriasis-Like Mouse Model
2.5.3. Histopathological Analysis
2.5.4. MPO Assay
2.6. In Vitro Experiments to Study Anti-Inflammatory Potential of WSSO
2.6.1. In Vitro Cell Culture
2.6.2. Cell Viability Study
2.6.3. Reactive Nitrogen Species (RNS) Measurement
2.6.4. Cytokines Level Measurement
2.6.5. Luciferase Reporter NFκB Expression Assay
2.7. Statistical Analysis
3. Results
3.1. SCFE Extraction of WS Seeds by CO2 and Analysis of Fatty Acid Contents by GC-FID
3.2. WSSO Contains Trace Amount of Withanolides
3.3. In Vivo Anti-Psoriatic Activity of WSSO
3.3.1. WSSO Inhibits Ear Edema in TPA Induced Psoriatic Mouse
3.3.2. WSSO Reduces Psoriatic Ear Epidermal Thickness and Weight
3.3.3. WSSO Reduces MPO Activity in Ear Biopsy Tissue
3.3.4. WSSO Repairs Psoriatic-Like Skin Lesions
3.4. In Vitro Anti-Inflammatory Activity of WSSO
3.4.1. WSSO Inhibits Psoriatic Inflammation in Human Epidermoid Cells
3.4.2. WSSO Inhibits Pro-Inflammatory Cytokines in LPS-Induced THP-1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulkarni, S.K.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Winters, M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern. Med. Rev. 2006, 11, 269–277. [Google Scholar]
- Ministry for Health and Family Welfare, Government of India. The Unani Pharmacopoeia of India; Pharmacopoeia Commission for Indian Medicine & Homoeopathy: Uttar Pradesh, India, 2007.
- Zhu, Y.P.; Woerdenbag, H.J. Traditional chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Grunz-Borgmann, E.; Mossine, V.; Fritsche, K.; Parrish, A.R. Ashwagandha attenuates TNF-α and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells. BMC Complement. Altern. Med. 2015, 15, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojha, S.; Alkaabi, J.; Amir, N.; Sheikh, A.; Agil, A.; Fahim, M.A.; Adem, A. Withania coagulans fruit extract reduces oxidative stress and inflammation in kidneys of streptozotocin-induced diabetic rats. Oxid. Med. Cell. Longev. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; DeWitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.S.; Nair, M.G. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phyther. Res. 2009, 23, 987–992. [Google Scholar] [CrossRef]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Vanden Berghe, T.; Vanden Berghe, W. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Effects of Ashwagandha (Roots of Withania somnifera) on neurodegenerative diseases. Biol. Pharm. Bull. 2014, 37, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, R.; Gupta, S.K.; Singh, N.; Mathur, S.; Kochupillai, V.; Velpandian, T. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J. Ethnopharmacol. 2006, 105, 336–341. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Al Hilo, A.S.; Al-Balas, Q.A.; El-Salem, K.; El-Elimat, T.; Alali, F.Q. Withania somnifera root powder protects againist post-traumatic stress disorder-induced memory impairment. Mol. Biol. Rep. 2019, 46, 4709–4715. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Singh, R.; Gao, R.; Shah, N.; Widodo, N.; Nakamoto, T.; Ishida, Y.; Terao, K.; Kaul, S.C. Water Extract of Ashwagandha Leaves Has Anticancer Activity: Identification of an Active Component and Its Mechanism of Action. PLoS ONE 2013, 8, e77189. [Google Scholar] [CrossRef]
- Shah, N.; Singh, R.; Sarangi, U.; Saxena, N.; Chaudhary, A.; Kaur, G.; Kaul, S.C.; Wadhwa, R. Combinations of Ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation. PLoS ONE 2015, 10, e0120554. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, P.; Kumar, A.; Ahuja, A.; Kaul, M. Fatty acid composition of seed oil of Withania somnifera selectants. Physiol. Mol. Biol. Plants 2007, 13, 73–76. [Google Scholar]
- Iguchi, T.; Kuroda, M.; Ishihara, M.; Sakagami, H.; Mimaki, Y. Steroidal constituents isolated from the seeds of Withania somnifera. Nat. Prod. Res. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Foster, R.H.; Hardy, G.; Alany, R.G. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718. [Google Scholar] [CrossRef]
- Liang, C.Y.; Lin, T.Y.; Lin, Y.K. Successful treatment of pediatric nail psoriasis with periodic pustular eruption using topical indigo naturalis oil extract. Pediatr. Dermatol. 2013, 30, 117–119. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Farber, E.M. The prevalence of psoriasis in the world. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 16–17. [Google Scholar] [CrossRef]
- Elder, J.T.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Stuart, P.E.; Tejasvi, T.; Voorhees, J.J.; Abecasis, G.R.; Nair, R.P. Molecular dissection of psoriasis: Integrating genetics and biology. J. Invest. Dermatol. 2010, 130, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, G.; Mignogna, C. The histopathology of psoriasis. Reumatismo 2007, 59, 46–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, J.G.; Bowcock, A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii30–ii36. [Google Scholar]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havnaer, A.; Weinberg, J.M.; Han, G. Systemic therapies in psoriasis: An update on newly approved and pipeline biologics and oral treatments. Cutis 2019, 104, 17–20. [Google Scholar] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmamoun, M.; Chandran, V. Role of Methotrexate in the Management of Psoriatic Arthritis. Drugs 2018, 78, 611–619. [Google Scholar] [CrossRef]
- Colombo, M.D.; Cassano, N.; Bellia, G.; Vena, G.A. Cyclosporine regimens in plaque psoriasis: An overview with special emphasis on dose, duration, and old and new treatment approaches. Sci. World J. 2013, 2013, 805705. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Han, J.; Qureshi, A.A. Use of aspirin, Non-steroidal anti-inflammatory drugs, and acetaminophen (paracetamol), And risk of psoriasis and psoriatic arthritis: A cohort study. Acta Derm. Venereol. 2015, 95, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Grösch, S.; Niederberger, E.; Geisslinger, G. Investigational drugs targeting the prostaglandin E2 signaling pathway for the treatment of inflammatory pain. Expert Opin. Investig. Drugs 2017, 26, 51–61. [Google Scholar] [CrossRef]
- Ernst, E. The efficacy of herbal medicine--an overview. Fundam. Clin. Pharmacol. 2005, 19, 405–409. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Nicolaou, A.; Harwood, J.L. Skin lipids in health and disease. Lipid Technol. 2016, 28, 36–39. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preparation of Methyl Esters of Fatty Acids (Ce 2-66), 7th ed.; AOCS Press: Urbana, IL, USA, 2017.
- Goto, M.; Kadoshima-Yamaoka, K.; Murakawa, M.; Yoshioka, R.; Tanaka, Y.; Inoue, H.; Murafuji, H.; Kanki, S.; Hayashi, Y.; Nagahira, K.; et al. Phosphodiesterase 7A inhibitor ASB16165 impairs proliferation of keratinocytes in vitro and in vivo. Eur. J. Pharmacol. 2010, 633, 93–97. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Joshi, K.; Sharma, V.; Ranjan, R.; Bhattacharya, K.; Varshney, A. Cytokines driven anti-inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (hippophae rhamnoides) oil. Front. Pharmacol. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Mehta, C.; Dave, A.; Shukla, V. Comparative effect of Navayasa Rasayana Leha and Medhya Rasayana tablet along with Dhatryadhyo Lepa in Ekkushtha (psoriasis). AYU (An. Int. Q. J. Res. Ayurveda) 2013, 34, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Kar, P.K.; Snehi, P.S.; Jha, P. Treatment of vitiligo with psoralen. Indian J. Dermatol. Venereol. Leprol. 1990, 56, 121–122. [Google Scholar]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.; Eng, P.A.; Borelli, S.; Kägi, R.; Zimmermann, C.; Zahner, C.; Drewe, J.; Hess, L.; Ferrari, G.; Lautenschlager, S.; et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv. Ther. 2014, 31, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Maeß, M.B.; Wittig, B.; Cignarella, A.; Lorkowski, S. Reduced PMA enhances the responsiveness of transfected THP-1 macrophages to polarizing stimuli. J. Immunol. Methods 2014, 402, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Azechi, K.; Mori, H. Identification of a novel protein kinase a inhibitor by bioluminescence-based screening. Biol. Pharm. Bull. 2015, 38, 1969–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Dhadde, S.B.; Vandal, R.; Shivakumar, B.S.; Charan, C.S. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: A systematic review and meta-analysis. J. Pharm. Pharmacol. 2015, 67, 879–899. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.K.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.N.; Rangnekar, V.M.; Gupta, R.C.; Bondada, S. Anti-cancer activity of withaferin A in B-cell lymphoma. Cancer Biol. Ther. 2015, 16, 1088–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; Hammers, H.; Bargagna-Mohan, P.; Zhan, X.; Herbstritt, C.; Ruiz, A.; Zhang, L.; Hanson, A.; Conner, B.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Yadav, B.; Bajaj, A.; Saxena, M.; Saxena, A.K. In Vitro anticancer activity of the root, stem and leaves of Withania Somnifera against various human cancer cell lines. Indian J. Pharm. Sci. 2010, 72, 659–663. [Google Scholar] [CrossRef] [Green Version]
- McHugh, M.A.; Krukonis, V.J. Supercritical fluid extraction: Principles and practice; Butterworth-Heinemann: Oxford, UK, 1994; ISBN 9781591241799. [Google Scholar]
- Mukhopadhyay, M. Natural Extracts Using Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9781420041699. [Google Scholar]
- Al-Otoom, A.; Al-Asheh, S.; Allawzi, M.; Mahshi, K.; Alzenati, N.; Banat, B.; Alnimr, B. Extraction of oil from uncrushed olives using supercritical fluid extraction method. J. Supercrit. Fluids 2014, 95, 512–518. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, M.K.; Jin, X.J.; Oh, J.H.; Kim, J.E.; Chung, J.H. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J. Korean Med. Sci. 2010, 25, 980–983. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.S.; Sun, H.L.; Lii, C.K.; Chen, H.W.; Chen, P.Y.; Liu, K.L. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-κB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the Ω-6 and Ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivamani, R.K. Eicosanoids and Keratinocytes in Wound Healing. Adv. Wound Care 2014, 3, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef]
- Souza, C.O.; Teixeira, A.A.S.; Biondo, L.A.; Silveira, L.S.; Calder, P.C.; Rosa Neto, J.C. Palmitoleic acid reduces the inflammation in LPS-stimulated macrophages by inhibition of NFκB, independently of PPARs. Clin. Exp. Pharmacol. Physiol. 2017, 44, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.R.; Burger, B.; Kühl, C.M.C.; Candreva, T.; dos Anjos, M.B.P.; Rodrigues, H.G. Wound healing and omega-6 fatty acids: From inflammation to repair. Mediators Inflamm. 2018, 2018, 2503950. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Sano, S. Mouse models of psoriasis and their relevance. J. Dermatol. 2018, 45, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.Y.; Soler, D.C.; Debanne, S.M.; Grozdev, I.; Rodriguez, M.E.; Feig, R.L.; Carman, T.L.; Gilkeson, R.C.; Orringer, C.E.; Kern, E.F.; et al. Psoriasis and cardiovascular risk factors: Increased serum myeloperoxidase and corresponding immunocellular overexpression by Cd11b+ CD68+ macrophages in skin lesions. Am. J. Transl. Res. 2014, 6, 16–27. [Google Scholar]
- Noske, K. Secreted immunoregulatory proteins in the skin. J. Dermatol. Sci. 2018, 89, 3–10. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Campanati, A.; Paolinelli, M.; Diotallevi, F.; Martina, E.; Molinelli, E.; Offidani, A. Pharmacodynamics of TNF-α inhibitors for the treatment of psoriasis. Expert Opin. Drug Metab. Toxicol. 2019, 15, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, W.; Herz, S.; Sody, E.; Körber, A. Dermatological complications of therapy with biologics in inflammatory autoimmune diseases. J. Dtsch. Dermatol. Ges. 2019, 17, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R.; Brooks, J.A.; Butte, N.F.; Caballero, B.; Flatt, J.P.; Fried, S.K. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academy Press: Washington, DC, USA, 2002; Volume 5, pp. 589–768. [Google Scholar]



| Sample Name | Withanoside IV Rt 21.96 | Withaferin A Rt 31.86 | Withanoside V Rt 34.33 | Withanolide A Rt 38.65 | Withanone Rt 39.32 | Withanolide B Rt 46.58 | Total Withanolides |
|---|---|---|---|---|---|---|---|
| WSSO | ND | 0.022 | ND | 0.001 | ND | ND | 0.023 |
| Post SCFE Exhaust of WS Seeds | 0.807 | 0.359 | 0.744 | ND | ND | ND | 1.910 |






| Peak No. | WSSO Component | Mol. Formula | Chemical Structure | Retention Time (min) | Content (%) |
|---|---|---|---|---|---|
| 1 | Palmitic Acid (Hexadecanoic acid) | C16H32O2 |  | 28.995 | 12.64 |
| 2 | Stearic Acid (Octadecanoic Acid) | C18H36O2 |  | 37.061 | 4.07 |
| 3 | Oleic Acid (Cis-9-Octadecenoic Acid) | C18H34O2 |  | 38.329 | 23.16 |
| 4 | Linoleic Acid | C18H32O2 |  | 40.719 | 54.15 |
| 5 | 11,14,17-Ecosatrienoic Acid | C20H34O2 |  | 48.521 | 0.66 |
| 6 | Nervonic Acid | C24H46O2 |  | 52.753 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Nain, P.; Chauhan, A.; Sharma, N.; Gupta, A.; Ranjan, R.; Varshney, A. Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules 2020, 10, 185. https://doi.org/10.3390/biom10020185
Balkrishna A, Nain P, Chauhan A, Sharma N, Gupta A, Ranjan R, Varshney A. Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules. 2020; 10(2):185. https://doi.org/10.3390/biom10020185
Chicago/Turabian StyleBalkrishna, Acharya, Pradeep Nain, Anshul Chauhan, Niti Sharma, Abhishek Gupta, Ravikant Ranjan, and Anurag Varshney. 2020. "Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release" Biomolecules 10, no. 2: 185. https://doi.org/10.3390/biom10020185
APA StyleBalkrishna, A., Nain, P., Chauhan, A., Sharma, N., Gupta, A., Ranjan, R., & Varshney, A. (2020). Super Critical Fluid Extracted Fatty Acids from Withania somnifera Seeds Repair Psoriasis-Like Skin Lesions and Attenuate Pro-Inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules, 10(2), 185. https://doi.org/10.3390/biom10020185