Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cell Cytotoxicity Assay
2.4. Cell Cycle Analysis
2.5. DAPI Staining
2.6. Annexin V/PI Double Staining Assay
2.7. Mitochondrial Membrane Potential Measurement
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity of Asiatic acid on Cisplatin-Resistance Human NPC Cell Lines
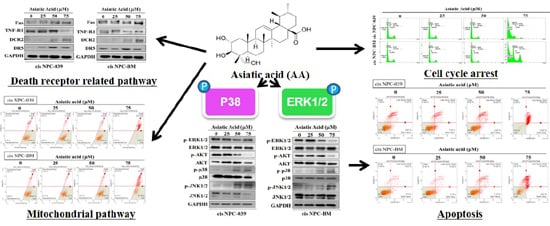
3.2. Asiatic Acid Induced Cell Apoptosis and Cell Cycle Arrest in Cisplatin-Resistance Human NPC Cell Lines
3.3. Asiatic Acid Induced Cell Apoptosis in Cisplatin-Resistance Human NPC Cell Lines through the Mitochondrial Pathway and Death Receptor Related Pathway
3.4. Asiatic Acid Upregulates the Expression of Caspase-3, -8, -9, and Altered the Expression of Bax and Bak in Cisplatin-Resistance Human NPC-039 and NPC-BM Cell Lines
3.5. Asiatic Acid Induced Cell Apoptosis via p38 Pathway in Cisplatin-Resistance Human NPC-039 and NPC-BM Cell Lines
4. Discussion
5. Conclusions
Data Availability Statement
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Paiar, F.; Di Cataldo, V.; Zei, G.; Pasquetti, E.M.; Cecchini, S.; Meattini, I.; Mangoni, M.; Agresti, B.; Iermano, C.; Bonomo, P.; et al. Role of chemotherapy in nasopharyngeal carcinoma. Oncol. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeran, M.F.N.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9. [Google Scholar]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Experiment. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef]
- Cui, Q.; Ren, J.; Zhou, Q.; Yang, Q.; Li, B. Effect of asiatic acid on epithelial-mesenchymal transition of human alveolar epithelium A549 cells induced by TGF-beta1. Oncol. Lett. 2019, 17, 4285–4292. [Google Scholar]
- Loong, H.H.; Ma, B.B.; Chan, A.T. Update on the Management and Therapeutic Monitoring of Advanced Nasopharyngeal Cancer. Hematol. Clin. North Am. 2008, 22, 1267–1278. [Google Scholar] [CrossRef]
- Su, W.-C.; Kao, R.-H.; Tsao, C.-J.; Chen, T.-Y. Chemotherapy with Cisplatin and Continuous Infusion of 5-Fluorouracil and Bleomycin for Recurrent and Metastatic Nasopharyngeal Carcinoma in Taiwan. Oncol. 1993, 50, 205–208. [Google Scholar] [CrossRef]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef]
- Siddique, A.I.; Mani, V.; Renganathan, S.; Ayyanar, R.; Nagappan, A.; Namasivayam, N. Asiatic acid abridges pre-neoplastic lesions, inflammation, cell proliferation and induces apoptosis in a rat model of colon carcinogenesis. Chem. Interactions 2017, 278, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jing, Y.; Cao, L.; Gong, C.; Gong, Z.; Cao, X. A novel synthetic Asiatic acid derivative induces apoptosis and inhibits proliferation and mobility of gastric cancer cells by suppressing STAT3 signaling pathway. OncoTargets Ther. 2017, 10, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakor, F.K.; Wan, K.-W.; Welsby, P.J.; Welsby, G. Pharmacological effects of asiatic acid in glioblastoma cells under hypoxia. Mol. Cell. Biochem. 2017, 430, 179–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharmaceutica Sinica 2017, 7, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Lv, T.; Chen, Y.; Wen, L.; Zhang, J.; Jiang, X.; Liu, F. Apoptosis of HL-60 human leukemia cells induced by Asiatic acid through modulation of B-cell lymphoma 2 family proteins and the mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2015, 12, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Lian, G.-Y.; Wang, Q.-M.; Tang, P.M.-K.; Zhou, S.; Huang, X.-R.; Lan, H.-Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018, 26, 2255–2266. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Cao, Q.-X.; Zhai, F.-R.; Yang, S.-Q.; Zhang, H.-X. Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm. Boil. 2016, 54, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Sakonsinsiri, C.; Kaewlert, W.; Armartmuntree, N.; Thanan, R.; Pakdeechote, P. Anti-cancer activity of asiatic acid against human cholangiocarcinoma cells through inhibition of proliferation and induction of apoptosis. Cell. Mol. Boil. 2018, 64, 28–33. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, K.; Bae, S.; Choi, Y.; Cha, H.J.; Kim, S.Y.; Lee, J.H.; Jeon, S.H.; Jung, H.J.; Ahn, K.J.; et al. MicroRNA-1290 promotes asiatic acidinduced apoptosis by decreasing BCL2 protein level in A549 nonsmall cell lung carcinoma cells. Oncol. Rep. 2014, 32, 1029–1036. [Google Scholar] [CrossRef]
- Gonçalves, B.M.; Salvador, J.A.R.; Marín, S.; Cascante, M. Synthesis and anticancer activity of novel fluorinated asiatic acid derivatives. Eur. J. Med. Chem. 2016, 114, 101–117. [Google Scholar] [CrossRef]
- Planchard, D.; Camara-Clayette, V.; Dorvault, N.; Soria, J.-C.; Fouret, P. p38 mitogen-activated protein kinase signaling, ERCC1 expression, and viability of lung cancer cells from never or light smoker patients. Cancer 2012, 118, 5015–5025. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223–8230. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFkappaB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-J.; Wang, C.-W.; Lin, J.-T.; Chuang, Y.-C.; Hsi, Y.-T.; Lo, Y.-S.; Lin, C.-C.; Chen, M.-K. Celastrol, a plant-derived triterpene, induces cisplatin-resistance nasopharyngeal carcinoma cancer cell apoptosis though ERK1/2 and p38 MAPK signaling pathway. Phytomedicine 2018, 58, 152805. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-T.; Chuang, Y.-C.; Lo, Y.-S.; Lin, C.-C.; Hsi, Y.-T.; Hsieh, M.-J.; Chen, M.-K. Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules 2020, 10, 184. https://doi.org/10.3390/biom10020184
Liu Y-T, Chuang Y-C, Lo Y-S, Lin C-C, Hsi Y-T, Hsieh M-J, Chen M-K. Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules. 2020; 10(2):184. https://doi.org/10.3390/biom10020184
Chicago/Turabian StyleLiu, Yen-Tze, Yi-Ching Chuang, Yu-Sheng Lo, Chia-Chieh Lin, Yi-Ting Hsi, Ming-Ju Hsieh, and Mu-Kuan Chen. 2020. "Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells" Biomolecules 10, no. 2: 184. https://doi.org/10.3390/biom10020184
APA StyleLiu, Y. -T., Chuang, Y. -C., Lo, Y. -S., Lin, C. -C., Hsi, Y. -T., Hsieh, M. -J., & Chen, M. -K. (2020). Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules, 10(2), 184. https://doi.org/10.3390/biom10020184