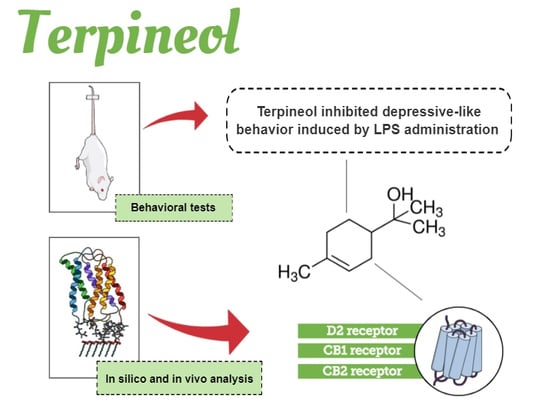
Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. ADMET Properties and Molecular Docking
2.3. Animals
2.4. Lipopolysaccharide (LPS)
2.5. Experimental Design
2.6. Tail Suspension Test (TST)
2.7. Splash Test (ST)
2.8. Open Field Test (OFT)
2.9. Statistical Analysis
3. Results and Discussion
3.1. ADMET Analysis
3.2. Molecular Modeling of D2 Dopaminergic Receptor and CB1/CB2 Cannabinoid Receptors by Terpineol
3.3. Evaluation of Terpineol’s Antidepressant-Like Effect in the TST, ST, and OFT
3.4. Antidepressant-Like Effect of Terpineol is Not Dependent on the A1 and A2A Adenosine Receptor Signaling Pathway
3.5. Involvement of Dopaminergic Receptors in Terpineol’s Antidepressant-Like Effects
3.6. Role of Cannabinoid Receptor Signaling Pathway in the Antidepressant-Like Effect Caused by Terpineol
3.7. Involvement of β-Adrenoceptor in Terpineol’s Antidepressant-Like Effects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- McLaughlin, K.A. The Public Health Impact of Major Depression: A Call for Interdisciplinary Prevention Efforts. Prev. Sci. 2011, 12, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C. The Costs of Depression. Psychiatr. Clin. North. Am. 2011, 35, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R. Psychoneuroimmunology of Depression: Clinical Implications. Brain, Behav. Immun. 2002, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, S.M.; Irwin, M.R. Depression and Immunity: Inflammation and Depressive Symptoms in Multiple Sclerosis. Neurol. Clin. 2006, 24, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol. Res. Perspect. 2019, 7, 1–20. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015, 16, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Tzavara, E.T.; Davis, R.J.; Perry, K.W.; Li, X.; Salhoff, C.; Bymaster, F.P.; Witkin, J.M.; Nomikos, G.G. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: Implications for therapeutic actions. Br. J. Pharmacol. 2003, 138, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Ming, Z.; Sawicki, G.; Bekar, L.K. Acute systemic LPS-mediated inflammation induces lasting changes in mouse cortical neuromodulation and behavior. Neurosci. Lett. 2015, 590, 96–100. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; Fronza, M.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Seixas, F.; Collares, T.; Nogueira, C.W.; Savegnago, L. Antidepressant-like effect of a new selenium-containing compound is accompanied by a reduction of neuroinflammation and oxidative stress in lipopolysaccharide-challenged mice. J. Psychopharmacol. 2017, 31, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Lawson, M.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2008, 14, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Mao, M.; Li, S.; Zhang, L.; Qiu, L.; Li, B.; Xia, J.; Yang, J. Acute ketamine administration attenuates lipopolysaccharide-induced depressive-like behavior by reversing abnormal regional homogeneity in the nucleus accumbens. NeuroReport 2019, 30, 421–427. [Google Scholar] [CrossRef]
- Cordeiro, R.C.; Filho, A.J.M.C.; Gomes, N.S.; Tomaz, V.D.S.; Medeiros, C.D.; Queiroz, A.I.D.G.; Maes, M.; Macedo, D.S.; Carvalho, A.F. Leptin Prevents Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice: Involvement of Dopamine Receptors. Front. Psychol. 2019, 10, 125–137. [Google Scholar] [CrossRef]
- Taniguti, E.; Ferreira, Y.; Stupp, I.; Fraga-Junior, E.; Doneda, D.; Lopes, L.; Rios-Santos, F.; Lima, E.; Buss, Z.; Viola, G.; et al. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res. Bull. 2019, 146, 279–286. [Google Scholar] [CrossRef]
- Remus, J.L.; Dantzer, R. Inflammation Models of Depression in Rodents: Relevance to Psychotropic Drug Discovery. Int. J. Neuropsychopharmacol. 2016, 19, 1–13. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Quintans-Júnior, L.J.; Oliveira, M.G.; De Santana, M.F.; Santana, M.T.; Guimarães, A.; Siqueira, J.S.; De Sousa, D.P.; Almeida, R.N. α-Terpineol reduces nociceptive behavior in mice. Pharm. Boil. 2011, 49, 583–586. [Google Scholar] [CrossRef]
- Trinh, H.-T.; Lee, I.-A.; Hyun, Y.-J.; Kim, N.-H. Artemisia princeps Pamp. Essential oil and its constituents eucalyptol and α-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-κB activation. Planta Medica 2011, 77, 1996–2002. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. The Roles of Vasopressin and Oxytocin in Memory Processing 2017, 80, 67–134. [Google Scholar] [CrossRef]
- Nogueira, M.N.M.; De Aquino, S.G.; Junior, C.R.; Spolidorio, D.M.P. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.G.; De Brito, R.G.; Santos, P.L.; Filho, H.G.D.A.; Quintans-Júnior, L.J.; Menezes, P.P.; Serafini, M.R.; Carvalho, Y.; Silva, J.C.; Almeida, J.R.G.D.S.; et al. α-Terpineol, a monoterpene alcohol, complexed with β-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem. Interactions 2016, 254, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Parvardeh, S.; Moghimi, M.; Eslami, P.; Masoudi, A. α-Terpineol attenuates morphine-induced physical dependence and tolerance in mice: Role of nitric oxide. Iran. J. Basic Med. Sci 2016, 19, 201–208. [Google Scholar] [PubMed]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Binfaré, R.W.; Mantovani, M.; Budni, J.; Dos Santos, A.R.S.; Rodrigues, A.L.S. Involvement of dopamine receptors in the antidepressant-like effect of melatonin in the tail suspension test. Eur. J. Pharmacol. 2010, 638, 78–83. [Google Scholar] [CrossRef]
- Safaripour, S.; Nemati, Y.; Parvardeh, S.; Ghafghazi, S.; Fouladzadeh, A.; Moghimi, M. Role of l -arginine/SNAP/NO/cGMP/KATP channel signalling pathway in antinociceptive effect of α-terpineol in mice. J. Pharm. Pharmacol. 2018, 70, 507–515. [Google Scholar] [CrossRef]
- Cosenza, M.; Gifford, A.N.; Gatley, S.J.; Pyatt, B.; Liu, Q.; Makriyannis, A.; Volkow, N.D. Locomotor activity and occupancy of brain cannabinoid CB1 receptors by the antagonist/inverse agonist AM281. Synap. 2000, 38, 477–482. [Google Scholar] [CrossRef]
- Paszcuk, A.F.; Dutra, R.C.; Da Silva, K.A.B.S.; Quintão, N.L.M.; Campos, M.M.; Calixto, J.B. Cannabinoid Agonists Inhibit Neuropathic Pain Induced by Brachial Plexus Avulsion in Mice by Affecting Glial Cells and MAP Kinases. PLoS ONE 2011, 6, 1–16. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.; Calixto, J.B. Beta-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARgamma pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular Docking. Advanced Structural Safety Studies 2008, 443, 365–382. [Google Scholar] [CrossRef]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; LaPrairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.-H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Natature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.; Taylor, R. Development and validation of a genetic algorithm for flexible docking 1 1Edited by F.E. Cohen. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, U.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Boil. 2010, 8, 1–6. [Google Scholar] [CrossRef]
- McGrath, J.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Lieberknecht, V.; Cunha, M.; Junqueira, S.C.; Coelho, I.D.S.; De Souza, L.; Dos Santos, A.R.S.; Rodrigues, A.L.S.; Dutra, R.C.; Dafre, A.L. Antidepressant-like effect of pramipexole in an inflammatory model of depression. Behav. Brain Res. 2017, 320, 365–373. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Ferro, J.N.S.; Rabelo, T.K.; Oliveira, M.A.; Scotti, L.; Scotti, M.T.; Walker, C.I.B.; Barreto, E.O.; Júnior, L.J.Q.; Guimarães, A. Alcoholic monoterpenes found in essential oil of aromatic spices reduce allergic inflammation by the modulation of inflammatory cytokines. Nat. Prod. Res. 2018, 33, 1773–1777. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Sim, W.-C.; Choi, H.K.; Lee, S.; Lee, B.-H. α-Terpineol induces fatty liver in mice mediated by the AMP-activated kinase and sterol response element binding protein pathway. Food Chem. Toxicol. 2013, 55, 129–136. [Google Scholar] [CrossRef]
- Oliveira, M.G.B.; Marques, R.B.; De Santana, M.F.; Santos, A.B.D.; Brito, F.A.; Barreto, E.O.; De Sousa, D.P.; Almeida, F.R.C.; Badauê-Passos, D.; Antoniolli, A.R.; et al. α-Terpineol Reduces Mechanical Hypernociception and Inflammatory Response. Basic Clin. Pharmacol. Toxicol. 2012, 111, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K.; Singh, T.; Mishra, A.; Goel, R.K. Relative Safety of Different Antidepressants for Treatment of Depression in Chronic Epileptic Animals Associated with Depression. J. Epilepsy Res. 2017, 7, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomkowski, A.D.E.; Hammes, L.; Lin, J.; Calixto, J.B.; Dos Santos, A.R.S.; Rodrigues, A.L.S. Agmatine produces antidepressant-like effects in two models of depression in mice. NeuroReport 2002, 13, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.; Pértile, R.; Calixto, J.B.; Dos Santos, A.R.S.; Rodrigues, A.L.S. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: Evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine-nitric oxide pathway. Neurosci. Lett. 2003, 343, 1–4. [Google Scholar] [CrossRef]
- De Rezende, V.B.; Rosa, D.V.; Comim, C.M.; Magno, L.A.V.; Rodrigues, A.L.S.; Vidigal, P.; Jeromin, A.; Quevedo, J.; Silva, M.A.R. NCS-1 deficiency causes anxiety and depressive-like behavior with impaired non-aversive memory in mice. Physiol. Behav. 2014, 130, 91–98. [Google Scholar] [CrossRef]
- Lobato, K.R.; Binfaré, R.W.; Budni, J.; Da Rosa, A.; Dos Santos, A.R.S.; Rodrigues, A.L.S. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog. Neuro-Psychopharmacology Boil. Psychiatry 2008, 32, 994–999. [Google Scholar] [CrossRef]
- Freitas, A.E.; Budni, J.; Lobato, K.R.; Binfaré, R.W.; Machado, D.G.; Jacinto, J.; Veronezi, P.O.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: Evidence for the involvement of the monoaminergic system. Prog. Neuro-Psychopharmacology Boil. Psychiatry 2010, 34, 335–343. [Google Scholar] [CrossRef]
- Neves, L.M.S.; Gonçalves, E.C.D.; Cavalli, J.; Vieira, G.; Laurindo, L.R.; Simões, R.R.; Coelho, I.S.; Dos Santos, A.R.S.; Marcolino, A.M.; Cola, M.; et al. Photobiomodulation Therapy Improves Acute Inflammatory Response in Mice: The Role of Cannabinoid Receptors/ATP-Sensitive K+ Channel/p38-MAPK Signalling Pathway. Mol. Neurobiol. 2017, 55, 5580–5593. [Google Scholar] [CrossRef]
- Yan, H.-C.; Cao, X.; Das, M.; Zhu, X.-H.; Gao, T.-M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Abelaira, H.M.; Réus, G.Z.; Quevedo, J. Animal models as tools to study the pathophysiology of depression. Rev. Bras. de Psiquiatr. 2013, 35, S112–S120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, S.L.; Narboux-Nême, N.; Boutourlinsky, K.; Doly, S.; Maroteaux, L.; Information, P.E.K.F.C. Mice lacking the serotonin 5-HT 2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur. Neuropsychopharmacol. 2016, 26, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, D.G.; Cunha, M.; Neis, V.B.; Balen, G.O.; Colla, A.; Grando, J.; Brocardo, P.S.; Bettio, L.; Capra, J.C.; Rodrigues, A.L.S. Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol. Biochem. Behav. 2012, 103, 220–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive Compound Collections: From Design to Target Identification. Chem 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Hoque, S.U.; Chowdhury, M.S.; Paul, A.; Barua, J.; Zannat, S.S.; Hasan, M.; Das Gupta, S.; Barua, S.; Ahmed, S.; Kabir, M.S.H. In vivo analgesic effect of different extracts of Hopea odorata leaves in mice and in silico molecular docking and ADME/T property analysis of some isolated compounds from this plant. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 121–130. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Singh, J.; Chuaqui, C.E.; Boriack-Sjodin, P.; Lee, W.-C.; Pontz, T.; Corbley, M.J.; Cheung, H.-K.; Arduini, R.M.; Mead, J.N.; Newman, M.N.; et al. Successful shape-Based virtual screening: The discovery of a potent inhibitor of the type I TGFβ receptor kinase (TβRI). Bioorganic Med. Chem. Lett. 2003, 13, 4355–4359. [Google Scholar] [CrossRef]
- Rester, U. From virtuality to reality–Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective. Curr. Opin. drug Discov. Dev. 2008, 11, 559–568. [Google Scholar]
- Bow, E.W.; Rimoldi, J. The Structure–Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect. Med. Chem. 2016, 8, PMC.S32171–39. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mechan, A.O.; Fowler, A.; Seifert, N.; Rieger, H.; Wöhrle, T.; Etheve, S.; Wyss, A.; Schüler, G.; Colletto, B.; Kilpert, C.; et al. Monoamine reuptake inhibition and mood-enhancing potential of a specified oregano extract. Br. J. Nutr. 2010, 105, 1150–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, É.R.Q.; Maia, C.S.F.; Junior, E.A.F.; Melo, A.S.; Pinheiro, B.; Maia, J.G.S. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 2018, 212, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.J.; Pan, S.-Y. Antidepressant-like Effect of Citrus sinensis (L.) Osbeck Essential Oil and Its Main Component Limonene on Mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Haleva-Toledo, E.; Naim, M.; Zehavi, U.; Rouseff, R. Formation of α-terpineol in Citrus Juices, Model and Buffer Solutions. J. Food Sci. 1999, 64, 838–841. [Google Scholar] [CrossRef]
- Gouveia, D.; Costa, J.S.; Oliveira, M.A.; Rabelo, T.K.; Silva, A.; Carvalho, A.A.; Miguel-Dos-Santos, R.; Lauton-Santos, S.; Scotti, L.; Scotti, M.T.; et al. α-Terpineol reduces cancer pain via modulation of oxidative stress and inhibition of iNOS. Biomed. Pharmacother. 2018, 105, 652–661. [Google Scholar] [CrossRef]
- Moon, P.-D.; Choi, I.S.; Go, J.-H.; Lee, B.-J.; Kang, S.W.; Yoon, S.; Han, S.-J.; Nam, S.-Y.; Oh, H.-A.; Han, N.-R.; et al. Inhibitory Effects of BiRyuChe-Bang on Mast Cell-Mediated Allergic Reactions and Inflammatory Cytokines Production. Am. J. Chin. Med. 2013, 41, 1267–1282. [Google Scholar] [CrossRef]
- Soleimani, M.; Sheikholeslami, M.A.; Ghafghazi, S.; Pouriran, R.; Parvardeh, S. Analgesic effect of α-terpineol on neuropathic pain induced by chronic constriction injury in rat sciatic nerve: Involvement of spinal microglial cells and inflammatory cytokines. Iran. J. Basic Med. Sci 2019, 22, 1445–1451. [Google Scholar]
- Liu, S.; Zhao, Y.; Cui, H.F.; Cao, C.Y.; Zhang, Y.B. 4-Terpineol exhibits potent in vitro and in vivo anticancer effects in Hep-G2 hepatocellular carcinoma cells by suppressing cell migration and inducing apoptosis and sub-G1 cell cycle arrest. J. Buon 2016, 21, 1195–1202. [Google Scholar]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J. Biomol. Struct. Dyn. 2019, 38, 2402–2411. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Daglia, M.; Jafari, S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016, 68, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Chiu, G.S.; Freund, G.G. Modulation of neuroimmunity by adenosine and its receptors: Metabolism to mental illness. Metab. 2014, 63, 1491–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Kobayashi, M.; Kanda, T. Involvement of Adenosine A2A Receptors in Depression and Anxiety. International Review of Neurobiology 2014, 119, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.R.; Hanff, T.C. Adenosine signaling in reserpine-induced depression in rats. Behav. Brain Res. 2015, 286, 184–191. [Google Scholar] [CrossRef]
- Peana, A.T.; Rubattu, P.; Piga, G.G.; Fumagalli, S.; Boatto, G.; Pippia, P.; De Montis, M.G. Involvement of adenosine A1 and A2A receptors in (−)-linalool-induced antinociception. Life Sci. 2006, 78, 2471–2474. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.H.; Yaoyao, J.; Jun, H.J.; Lee, S.-J. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A2A receptors. Biochem. Biophys. Res. Commun. 2011, 404, 345–348. [Google Scholar] [CrossRef]
- Marks, D.M.; Pae, C.-U.; Patkar, A.A. Triple Reuptake Inhibitors: The Next Generation of Antidepressants. Curr. Neuropharmacol. 2008, 6, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhao, J.; Lv, J.; Tang, F.; Liu, L.; Sun, Z.; Wang, L.; Siwela, S.P.; Wang, Y.; Song, Y.; et al. Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog. Neuro-Psychopharmacology Boil. Psychiatry 2014, 48, 199–206. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, Y.; Li, G.; Wang, B.; Zhou, T.; Zhu, L.; Chen, T.; Chen, Y. The Dopamine Receptor D3 Regulates Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Int. J. Neuropsychopharmacol. 2018, 21, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Cansino, R.G.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, I.; Abdelhalim, A.; Khan, A.; Halim, S.A. Antidepressant potential of novel flavonoids derivatives from sweet violet (Viola odorata L): Pharmacological, biochemical and computational evidences for possible involvement of serotonergic mechanism. Fitoter. 2018, 128, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Maleki, S.; Mousavi, Z. Hydroethanolic extract of Carthamus tinctorius induces antidepressant-like effects: Modulation by dopaminergic and serotonergic systems in tail suspension test in mice. Iran. J. Basic Med. Sci 2017, 20, 1063–1073. [Google Scholar] [PubMed]
- Umukoro, S.; Adebesin, A.; Agu, G.; Omorogbe, O.; Asehinde, S.B. Antidepressant-like activity of methyl jasmonate involves modulation of monoaminergic pathways in mice. Adv. Med. Sci. 2017, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Takeuchi, T.; Harada, E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006, 172, 240–249. [Google Scholar] [CrossRef]
- Bassi, M.S.; Gilio, L.; Maffei, P.; Dolcetti, E.; Bruno, A.; Buttari, F.; Centonze, D.; Iezzi, E. Exploiting the Multifaceted Effects of Cannabinoids on Mood to Boost Their Therapeutic Use Against Anxiety and Depression. Front. Mol. Neurosci. 2018, 11, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Gorzalka, B.B.; Hill, M.N. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog. Neuro-Psychopharmacology Boil. Psychiatry 2011, 35, 1575–1585. [Google Scholar] [CrossRef]
- Hill, M.N.; Hillard, C.J.; Bambico, F.R.; Patel, S.; Gorzalka, B.B.; Gobbi, G. The Therapeutic Potential of the Endocannabinoid System for the Development of a Novel Class of Antidepressants. Trends Pharmacol. Sci. 2009, 30, 484–493. [Google Scholar] [CrossRef]
- Zoppi, S.; Madrigal, J.; Caso, J.R.; García-Gutiérrez, M.S.; Manzanares, J.; Leza, J.C.; Garcia-Bueno, B. Regulatory role of the cannabinoid CB2receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, T.; Maroso, M.; Beer, A.; Baddenhausen, S.; Ludewig, S.; Fan, W.; Vennin, C.; Loch, S.; Berninger, B.; Hofmann, C.; et al. Neural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampus. Cereb. Cortex 2018, 28, 4454–4471. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Kadkhoda, Z.; Taghizad-Farid, R. The antidepressant-like effects of Origanum majorana essential oil on mice through monoaminergic modulation using the forced swimming test. J. Tradit. Complement. Med. 2019. [Google Scholar] [CrossRef]
- Coelho, V.; Mazzardo-Martins, L.; Martins, D.F.; Santos, A.R.; da Silva Brum, L.F.; Picada, J.N.; Pereira, P. Neurobehavioral and genotoxic evaluation of (−)-linalool in mice. J. Nat. Med. 2013, 67, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Moghaddam, A.H.; Maggi, F.; Benelli, G. Anxiolytic and antidepressant activities ofPelargonium roseumessential oil on Swiss albino mice: Possible involvement of serotonergic transmission. Phytotherapy Res. 2018, 32, 1014–1022. [Google Scholar] [CrossRef]
- Akbaba, E.; Hassan, S.; Sur, T.M.; Bagci, E. Memory Enhancing, Anxiolytic and Antidepressant Effects of Achillea biebersteinii (Asteraceae) Essential Oil on Scopolamine-Induced Rats. J. Essent. Oil Bear. Plants 2018, 21, 825–839. [Google Scholar] [CrossRef]
- Deyo, R.; Musty, R. A cannabichromene (CBC) extract alters behavioral despair on the mouse tail suspension test of depression. In Proceedings of the 13th Symposium on the cannabinoids, Cornwall, UK, 24 June 2003; International Cannabinoid Research Society: Cornwall, UK, 2003; p. 146. [Google Scholar]
- Musty, R.; Deyo, R. A cannabigerol extract alters behavioral despair in an animal model of depression. In Proceedings of the Symposium on the Cannabinoids, Vermont, USA, 24 June 2006; International Cannabinoid Research Society: Vermont, VT, USA, 2006; p. 32. [Google Scholar]
- Cascio, M.; Gauson, L.; Stevenson, L.; Ross, R.; Pertwee, R. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2009, 159, 129–141. [Google Scholar] [CrossRef] [Green Version]
- George, S.A.; Knox, D.; Curtis, A.L.; Aldridge, J.W.; Valentino, R.J.; Liberzon, I. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur. J. Neurosci. 2012, 37, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Pacak, K.; Palkovits, M.; Kvetnansky, R.; Yadid, G.; Kopin, I.; Goldstein, D.S. Effects of Various Stressors on In Vivo Norepinephrine Release in the Hypothalamic Paraventricular Nucleus and on the Pituitary-Adrenocortical Axis. Ann. New York Acad. Sci. 1995, 771, 115–130. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Seki, K.; Yoshida, S. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [CrossRef]









| Drugs | Dose | References |
|---|---|---|
| Haloperidol—nonselective dopaminergic receptor antagonist | 0.2 mg/kg (i.p.) | [25,38] |
| Sulpiride—selective dopamine D2 receptor antagonist | 50 mg/kg (i.p.) | [25,38] |
| Propranolol—β-adrenoceptor antagonist | 2 mg/kg (i.p.) | [48] |
| Caffeine—nonselective adenosine receptor antagonist | 3 mg/kg (i.p.) | [47] |
| AM281—selective CB1 receptor antagonist / inverse agonist | 1 mg/kg (i.p.) | [49] |
| AM630—selective inverse agonist for the CB2 receptor | 1 mg/kg (i.p.) | [49] |
| Model | Result | Probability |
|---|---|---|
| Absorption | ||
| Blood-Brain Barrier | BBB+ | 0.9923 |
| Human intestinal absorption | HIA+ | 0.9941 |
| Caco-2 permeability | Caco2+ | 0.8160 |
| Human oral bioavailability | + | 0.6857 |
| P-glycoprotein substrate | Non-substrate | 0.9405 |
| P-glycoprotein inhibitor | Non-inhibitor | 0.9843 |
| Renal organic cation transporter | Non-inhibitor | 0.8024 |
| Distribution | ||
| Subcellular localization | Lysosomes | 0.4268 |
| Metabolism | ||
| OATP1B1 inhibitor | Inhibitor | 0.9677 |
| OATP2B1 inhibitor | Non-inhibitor | 0.8466 |
| OATP1B3 inhibitor | Inhibitor | 0.8719 |
| MATE1 inhibitor | Non-inhibitor | 0.9600 |
| OCT2 inhibitor | Non-inhibitor | 0.6750 |
| BSEP inhibitor | Non-inhibitor | 0.9059 |
| CYP2D6 substrate | Non-substrate | 0.7630 |
| CYP3A4 inhibition | Non-inhibitor | 0.8411 |
| CYP2C19 inhibition | Non-inhibitor | 0.7049 |
| CYP2D6 inhibition | Non-inhibitor | 0.9322 |
| UGT catalyzed | + | 0.7000 |
| Toxicity | ||
| Ames mutagenesis | Non-AMES toxic | 0.9800 |
| Carcinogens | Non-carcinogens | 0.8429 |
| Acute oral toxicity | IV | 0.6381 |
| Carcinogenicity (trinary) | Non-required | 0.5811 |
| Hepatotoxicity | Non-toxic | 0.7750 |
| Eye corrosion | Non-toxic | 0.8463 |
| Biodegradation | + | 0.7750 |
| ADMET Predicted Profile–Regression | ||
| Water solubility | −2.336 LogS | - |
| Plasma protein binding | 0.652 (100%) | - |
| Acute oral toxicity | 2.137 kg/mol | - |
| Fish aquatic toxicity | 0.6781 pLC50, mg/L | - |
| Tetrahymena pyriformis | −0.468 pIGC50 (μg/L) | - |
| Rat acute toxicity | 1.5063 LD50, mol/kg | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, G.; Cavalli, J.; Gonçalves, E.C.D.; Braga, S.F.P.; Ferreira, R.S.; Santos, A.R.S.; Cola, M.; Raposo, N.R.B.; Capasso, R.; Dutra, R.C. Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules 2020, 10, 792. https://doi.org/10.3390/biom10050792
Vieira G, Cavalli J, Gonçalves ECD, Braga SFP, Ferreira RS, Santos ARS, Cola M, Raposo NRB, Capasso R, Dutra RC. Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules. 2020; 10(5):792. https://doi.org/10.3390/biom10050792
Chicago/Turabian StyleVieira, Graziela, Juliana Cavalli, Elaine C. D. Gonçalves, Saulo F. P. Braga, Rafaela S. Ferreira, Adair R. S. Santos, Maíra Cola, Nádia R. B. Raposo, Raffaele Capasso, and Rafael C. Dutra. 2020. "Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor" Biomolecules 10, no. 5: 792. https://doi.org/10.3390/biom10050792
APA StyleVieira, G., Cavalli, J., Gonçalves, E. C. D., Braga, S. F. P., Ferreira, R. S., Santos, A. R. S., Cola, M., Raposo, N. R. B., Capasso, R., & Dutra, R. C. (2020). Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules, 10(5), 792. https://doi.org/10.3390/biom10050792