Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry of Triazoles
2.2. Biological Assays
2.2.1. Maintenance of P. falciparum Culture
2.2.2. Maintenance of HEK293 Cell Culture
2.2.3. In Vitro Growth Assay and Flow Cytometry Analysis
2.2.4. Cytotoxicity Assay in HEK293 Cells
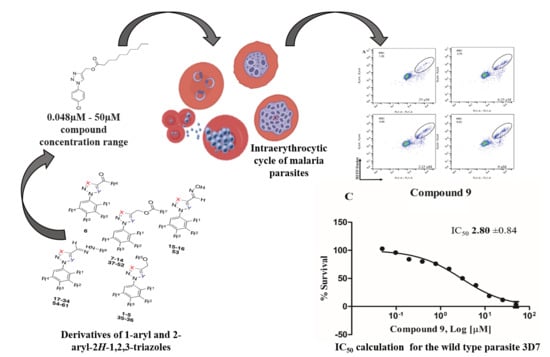
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solyakov, L.; Halbert, J.; Alam, M.M.; Semblat, J.P.; Dorin-Semblat, D.; Reininger, L.; Bottrill, A.R.; Mistry, S.; Abdi, A.; Fennell, C.; et al. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun. 2011, 2, 1–12. [Google Scholar] [CrossRef]
- WHO. Fact Sheet about Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 13 April 2020).
- Blasco, B.; Leroy, D.; Fidock, D.A. Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Ackerman, H.C.; Su, X.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Kirk, K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004, 21, 1938–1949. [Google Scholar] [CrossRef] [Green Version]
- Singh Sidhu, A.B.; Verdier-Pinard, D.; Fidock, D.A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 2002, 298, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Marchetti, R.V.; Cowan, A.I.; Howitt, S.M.; Bröer, S.; Kirk, K. Chloroquine Transport via the Malaria Parasite’s Chloroquine Resistance Transporter. Science 2009, 325, 1680–1682. [Google Scholar] [CrossRef] [Green Version]
- Summers, R.L.; Dave, A.; Dolstra, T.J.; Bellanca, S.; Marchetti, R.V.; Nash, M.N.; Richards, S.N.; Goh, V.; Schenk, R.L.; Stein, W.D.; et al. Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Proc. Natl. Acad. Sci. USA 2014, 111, E1759–E1767. [Google Scholar] [CrossRef] [Green Version]
- Juge, N.; Moriyama, S.; Miyaji, T.; Kawakami, M.; Iwai, H.; Fukui, T.; Nelson, N.; Omote, H.; Moriyama, Y.; Kaback, H.R. Plasmodium falciparum chloroquine resistance transporter is a H +-coupled polyspecific nutrient and drug exporter. Proc. Natl. Acad. Sci. USA 2015, 112, 3356–3361. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Artemisinin and Artemisinin-Based Combination Therapy Resistance: Status Report; World Health Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- Miotto, O.; Amato, R.; Ashley, E.A.; MacInnis, B.; Almagro-Garcia, J.; Amaratunga, C.; Lim, P.; Mead, D.; Oyola, S.O.; Dhorda, M.; et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat. Genet. 2015, 47, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neher, R.A.; MalariaGEN Plasmodium falciparum Community Project. Genomic epidemiology of artemisinin resistant malaria. Elife 2016. [Google Scholar] [CrossRef]
- Egan, T.J. Quinoline antimalarials. Expert Opin. Ther. Pat. 2001, 11, 185–209. [Google Scholar] [CrossRef]
- Bray, P.; Park, B.; Asadollaly, E.; Biagini, G.; Jeyadevan, J.; Berry, N.; Ward, S.; O’ Neill, P. A Medicinal Chemistry Perspective on 4-Aminoquinoline Antimalarial Drugs. Curr. Top. Med. Chem. 2006, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Maluf, F.V.; Bueno, V.B.; Guido, R.V.C.; Oliva, G.; Singh, M.; Scarpelli, P.; Costa, F.; Sartorello, R.; Catalani, L.H.; et al. Biliverdin targets enolase and eukaryotic initiation factor 2 (eIF2α) to reduce the growth of intraerythrocytic development of the malaria parasite Plasmodium falciparum. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigala, P.A.; Crowley, J.R.; Hsieh, S.; Henderson, J.P.; Goldberg, D.E. Direct tests of enzymatic heme degradation by the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2012, 287, 37793–37807. [Google Scholar] [CrossRef] [Green Version]
- Schuck, D.C.; Ferreira, S.B.; Cruz, L.N.; Da Rocha, D.R.; Moraes, M.S.; Nakabashi, M.; Rosenthal, P.J.; Ferreira, V.F.; Garcia, C.R.S. Biological evaluation of hydroxynaphthoquinones as anti-malarials. Malar. J. 2013, 12, 234. [Google Scholar] [CrossRef] [Green Version]
- Najahi, E.; Valentin, A.; Fabre, P.L.; Reybier, K.; Nepveu, F. 2-Aryl-3H-indol-3-ones: Synthesis, electrochemical behaviour and antiplasmodial activities. Eur. J. Med. Chem. 2014, 78, 269–274. [Google Scholar] [CrossRef]
- Schuck, D.C.; Jordão, A.K.; Nakabashi, M.; Cunha, A.C.; Ferreira, V.F.; Garcia, C.R.S. Synthetic indole and melatonin derivatives exhibit antimalarial activity on the cell cycle of the human malaria parasite Plasmodium falciparum. Eur. J. Med. Chem. 2014, 78, 375–382. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Wang, W.-L.; Liang, L.-L.; Liu, W.; Gong, K.-K.; Sun, K.-L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Xu, J.-H.; Fan, Y.-L.; Zhou, J. Quinolone–Triazole Hybrids and their Biological Activities. J. Heterocycl. Chem. 2018, 55, 1854–1862. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Therrien, C.; Levesque, R.C. Molecular basis of antibiotic resistance and β-lactamase inhibition by mechanism-based inactivators: Perspectives and future directions. FEMS Microbiol. Rev. 2000, 24, 251–262. [Google Scholar] [CrossRef]
- Boechat, N.; Ferreira, M.D.L.G.; Pinheiro, L.C.S.; Jesus, A.M.L.; Leite, M.M.M.; Júnior, C.C.S.; Aguiar, A.C.C.; De Andrade, I.M.; Krettli, A.U. New compounds hybrids 1H-1,2,3-triazole-quinoline against plasmodium falciparum. Chem. Biol. Drug Des. 2014, 84, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Saikrishn, B.; Kotni, M.; Manga, V.; Allanki, A.D.; Prasad, R.; Sijwali, P.S. Synthesis and evaluation of naphthyl bearing 1,2,3-triazole analogs as antiplasmodial agents, cytotoxicity and docking studies. Bioorg. Med. Chem. 2017, 25, 221–232. [Google Scholar]
- D’Hooghe, M.; Vandekerckhove, S.; Mollet, K.; Vervisch, K.; Dekeukeleires, S.; Lehougg, L.; Lategan, C.; Smith, P.J.; Chibale, K.; De Kimpe, N. Synthesis of 2-amino-3-arylpropan-1-ols and 1-(2,3-diaminopropyl)-1,2,3-triazoles and evaluation of their antimalarial activity. Beilstein J. Org. Chem. 2011, 7, 1745–1752. [Google Scholar] [CrossRef]
- Joshi, M.C.; Wicht, K.J.; Taylor, D.; Hunter, R.; Smith, P.J.; Egan, T.J. In vitro antimalarial activity, β-haematin inhibition and structure–activity relationships in a series of quinoline triazoles. Eur. J. Med. Chem. 2013, 69, 338–347. [Google Scholar] [CrossRef]
- Brandão, G.C.; Missias, F.C.R.; Arantes, L.M.; Soares, L.F.; Roy, K.K.; Doerksen, R.J.; Oliveira, A.B.; Pereira, G.R. Antimalarial naphthoquinones. Synthesis via click chemistry, in vitro activity, docking to PfDHODH and SAR of lapachol-based compounds. Eur. J. Med. Chem. 2018, 145, 191–205. [Google Scholar]
- Moraes, M.S.; Budu, A.; Singh, M.K.; Borges-Pereira, L.; Levano-Garcia, J.; Currà, C.; Picci, L.; Pace, T.; Ponzi, M.; Pozzan, T.; et al. Plasmodium falciparum GPCR-like receptor SR25 mediates extracellular K+ sensing coupled to Ca2+ signaling and stress survival. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Boechat, N.; Ferreira, V.F.; Ferreira, S.B.; Ferreira, M.D.L.G.; Da Silva, F.D.C.; Bastos, M.M.; Costa, M.D.S.; Lourenço, M.C.S.; Pinto, A.C.; Krettli, A.U.; et al. Novel 1,2,3-triazole derivatives for use against mycobacterium tuberculosis H37Rv (ATCC 27294) strain. J. Med. Chem. 2011, 54, 5988–5999. [Google Scholar] [CrossRef]
- Faria, R.X.; Gonzaga, D.T.G.; Pacheco, P.A.F.; Souza, A.L.A.; Ferreira, V.F.; da Silva, F.D.C. Searching for new drugs for Chagas diseases: Triazole analogs display high in vitro activity against Trypanosoma cruzi and low toxicity toward mammalian cells. J. Bioenerg. Biomembr. 2018, 50, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, D.; Senger, M.R.; Da Silva, F.D.C.; Ferreira, V.F.; Silva, F.P. 1-Phenyl-1H- and 2-phenyl-2H-1,2,3-triazol derivatives: Design, synthesis and inhibitory effect on alpha-glycosidases. Eur. J. Med. Chem. 2014, 74, 461–476. [Google Scholar] [CrossRef]
- Gonzaga, D.T.G.; Ferreira, L.B.G.; Costa, T.E.M.M.; von Ranke, N.L.; Pacheco, P.A.F.; Simões, A.P.S.; Arruda, J.C.; Dantas, L.P.; de Freitas, H.R.; de Melo Reis, R.A.; et al. 1-Aryl-1 H - and 2-aryl-2 H -1,2,3-triazole derivatives blockade P2X7 receptor in vitro and inflammatory response in vivo. Eur. J. Med. Chem. 2017, 139, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Ekland, E.H.; Schneider, J.; Fidock, D.A. Identifying apicoplast-targeting antimalarials using high-throughput compatible approaches. FASEB J. 2011, 25, 3583–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Garcia, C.R.S.; Takeuschi, M.; Yoshioka, K.; Miyamoto, H. Imaging Plasmodium falciparum-infected ghost and parasite by atomic force microscopy. J. Struct. Biol. 1997, 119, 92–98. [Google Scholar] [CrossRef]
- Witkowski, B.; Duru, V.; Khim, N.; Ross, L.S.; Saintpierre, B.; Beghain, J.; Chy, S.; Kim, S.; Ke, S.; Kloeung, N.; et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: A phenotype–genotype association study. Lancet Infect. Dis. 2017, 17, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Hotta, C.T.; Gazarini, M.L.; Beraldo, F.H.; Varotti, F.P.; Lopes, C.; Markus, R.P.; Pozzan, T.; Garcia, C.R.S. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat. Cell Biol. 2000, 2, 466–468. [Google Scholar] [CrossRef]
- Hotta, C.T.; Markus, R.P.; Garcia, C.R.S. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz. J. Med. Biol. Res. 2003, 36, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Ross, L.S.; Fidock, D.A. Elucidating Mechanisms of Drug-Resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Cowell, A.N.; Winzeler, E.A. The genomic architecture of antimalarial drug resistance. Brief. Funct. Genom. 2019, 18, 314–328. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, V.; Bray, P.G.; Verdier-Pinard, D.; Johnson, D.J.; Horrocks, P.; Muhle, R.A.; Alakpa, G.E.; Hughes, R.H.; Ward, S.A.; Krogstad, D.J.; et al. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 2005, 24, 2294–2305. [Google Scholar] [CrossRef]
- Ross, L.S.; Dhingra, S.K.; Mok, S.; Yeo, T.; Wicht, K.J.; Kümpornsin, K.; Takala-Harrison, S.; Witkowski, B.; Fairhurst, R.M.; Ariey, F.; et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 2018, 9, 3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, R.; Lim, P.; Miotto, O.; Amaratunga, C.; Dek, D.; Pearson, R.D.; Almagro-Garcia, J.; Neal, A.T.; Sreng, S.; Suon, S.; et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: A genotype–phenotype association study. Lancet Infect. Dis. 2017, 17, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Khanal, P.; Mandar, B.K.; Magadum, P.; Patil, B.M.; Hullatti, K.K. In silico docking study of Limonoids from Azadirachta indica with pfpk5: A Novel Target for Plasmodium falciparum. Indian J. Pharm. Sci. 2019, 81, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Z.Y.; Uzairu, A.; Shallangwa, G.; Abechi, S. QSAR and molecular docking based design of some indolyl-3-ethanone-α-thioethers derivatives as Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) inhibitors. SN Appl. Sci. 2020, 2, 117. [Google Scholar] [CrossRef]
- Iman, M.; Davood, A.; Khamesipour, A. Design of antimalarial agents based on pyrimidine derivatives as methionine aminopeptidase 1b inhibitor: Molecular docking, quantitative structure activity relationships, and molecular dynamics simulation studies. J. Chin. Chem. Soc. 2019, 1–11. [Google Scholar] [CrossRef]
- Madeira, L.; Galante, P.A.F.; Budu, A.; Azevedo, M.F.; Malnic, B.; Garcia, C.R.S. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [Green Version]






| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | X | Y | IC50 (μM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | H | Cl | H | Et | - | - | - | N | CH | 11.65 ± 0.74 |
| 2 | H | Cl | H | Cl | Et | - | - | - | N | CH | 15.41 ± 2.26 |
| 3 | H | Cl | H | Cl | Pr | - | - | - | N | CH | 14.52 ± 1.71 |
| 4 | Cl | H | H | Cl | Pr | - | - | - | N | CH | 20.45 ± 0.73 |
| 5 | H | Cl | H | Cl | Bu | - | - | - | N | CH | 23.94 ± 2.01 |
| 6 | H | H | H | H | - | OEt | - | - | N | CH | 12.84 ± 3.02 |
| 7 | H | H | H | H | - | - | Ph | - | N | CH | 22.93 ± 0.34 |
| 8 | H | H | H | H | - | - | Ph | - | CH | N | 27.49 ± 1.26 |
| 9 | H | H | Cl | H | - | - | nonyl | - | N | CH | 2.80 ± 0.84 |
| 10 | H | Cl | H | Cl | - | - | nonyl | - | N | CH | 14.64 ± 0.29 |
| 11 | H | H | OMe | H | - | - | nonyl | - | N | CH | 23.46 ± 2.77 |
| 12 | H | H | OMe | H | - | - | Ph | - | N | CH | 13.53 ± 1.12 |
| 13 | H | H | Cl | H | - | - | - | - | N | CH | 6.67 ± 1.86 |
| 14 | Cl | H | H | Cl | - | - | - | - | N | CH | 21.52 ± 0.84 |
| 15 | H | H | H | H | - | - | - | Ph | CH | N | 18.05 ± 1.92 |
| 16 | H | H | H | H | - | - | - | 4-Cl-Ph | CH | N | 9.29 ± 1.01 |
| 17 | H | H | H | H | - | - | - | 4-F-Ph | CH | N | 14.68 ± 1.28 |
| 18 | H | H | Cl | H | - | - | - | 4-Cl-Ph | N | CH | 17.61 ± 1.07 |
| 19 | H | H | Cl | H | - | - | - | 4-F-Ph | N | CH | 8.45 ± 2.25 |
| 20 | H | H | Cl | H | - | - | - | 2.5-diCl-Ph | N | CH | 13.58 ± 1.20 |
| 21 | H | Cl | H | Cl | - | - | - | 4-Br-Ph | N | CH | 29.27 ± 0.34 |
| 22 | H | Cl | H | Cl | - | - | - | 2,5-diCl-Ph | N | CH | 5.56 ± 0.47 |
| 23 | H | Cl | H | Cl | - | - | - | 2,5-diMe-Ph | N | CH | 9.23 ± 1.68 |
| 24 | H | H | OMe | H | - | - | - | Ph | N | CH | 4.44 ± 1.43 |
| 25 | H | H | OMe | H | - | - | - | 4-Cl-Ph | N | CH | 9.00 ± 3.27 |
| 26 | Cl | H | H | Cl | - | - | - | 2,5-diCl-Ph | N | CH | 9.40 ± 2.87 |
| 27 | H | H | H | H | - | - | - | -CO-4-pyridil | N | CH | 23.12 ± 2.58 |
| 28 | H | H | Cl | H | - | - | - | -CO-4-pyridil | N | CH | 14.52 ± 1.13 |
| 29 | H | Cl | H | Cl | - | - | - | -CO-4-pyridil | N | CH | 6.96 ± 1.44 |
| 30 | H | H | OMe | H | - | - | - | -CO-4-pyridil | N | CH | 23.20 ± 1.94 |
| 31 | H | H | H | H | - | - | - | -CO-4-pyridil | CH | N | 20.16 ± 0.79 |
| Compound | Molecular Structure | IC50 µM |
|---|---|---|
| 9 |  | 1.55 ± 0.55 |
| 13 |  | 0.89 ±0.39 |
| 19 |  | 2.24 ± 0.77 |
| 21 |  | 12.42 ± 2.51 |
| 22 |  | 4.41 ± 0.60 |
| 24 |  | 0.99 ± 0.25 |
| 29 |  | 9.91 ± 2.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, B.M.d.; Gonzaga, D.T.G.; da Silva, F.C.; Ferreira, V.F.; Garcia, C.R.S. Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules 2020, 10, 1197. https://doi.org/10.3390/biom10081197
Santos BMd, Gonzaga DTG, da Silva FC, Ferreira VF, Garcia CRS. Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules. 2020; 10(8):1197. https://doi.org/10.3390/biom10081197
Chicago/Turabian StyleSantos, Benedito M. dos, Daniel T. G. Gonzaga, Fernando C. da Silva, Vitor F. Ferreira, and Celia R. S. Garcia. 2020. "Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development" Biomolecules 10, no. 8: 1197. https://doi.org/10.3390/biom10081197
APA StyleSantos, B. M. d., Gonzaga, D. T. G., da Silva, F. C., Ferreira, V. F., & Garcia, C. R. S. (2020). Plasmodium falciparum Knockout for the GPCR-Like PfSR25 Receptor Displays Greater Susceptibility to 1,2,3-Triazole Compounds That Block Malaria Parasite Development. Biomolecules, 10(8), 1197. https://doi.org/10.3390/biom10081197