TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch
Abstract
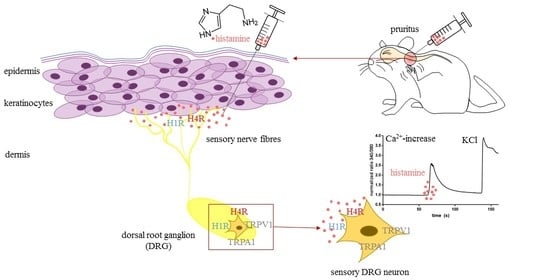
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Evaluation of Scratching Behavior
2.4. Isolation of DRG Neurons
2.5. Ca2+-Imaging
2.6. Statistics
3. Results
3.1. Effect of TRP Channels on Histamine-Induced Pruritus
3.2. Effect of TRP Channels on Histamine-Induced Intracellular Ca2+-Increase
3.3. Role of TRPV1 and TRPA1 on 4-MH-Induced Ca2+-Increase in DRG Neurons in Three Different Mouse Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.K.; McQueen, D.S.; Rees, J.L. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in BalbC mice. Br. J. Pharmacol. 2004, 142, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossbach, K.; Nassenstein, C.; Gschwandtner, M.; Schnell, D.; Sander, K.; Seifert, R.; Stark, H.; Kietzmann, M.; Bäumer, W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 2011, 190, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Zotterman, Y. Vascular reactions of the skin to injury: Part VIII. The resistance of the human skin to constant currents, in relation to injury and vascular response. J. Physiol. 1927, 62, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, H.; Tominaga, M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol. Int. 2017, 66, 22–30. [Google Scholar] [CrossRef]
- Han, S.-K.; Mancino, V.; Simon, M.I. Phospholipase Cβ 3 Mediates the Scratching Response Activated by the Histamine H1 Receptor on C-Fiber Nociceptive Neurons. Neuron 2006, 52, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Jian, T.; Yang, N.; Yang, Y.; Zhu, C.; Yuan, X.; Yu, G.; Wang, C.; Wang, Z.; Shi, H.; Tang, M.; et al. TRPV1 and PLC Participate in Histamine H4 Receptor-Induced Itch. Neural Plast. 2015, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kajihara, Y.; Murakami, M.; Imagawa, T.; Otsuguro, K.; Ito, S.; Ohta, T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience 2010, 166, 292–304. [Google Scholar] [CrossRef]
- Kim, B.M.; Lee, S.H.; Shim, W.S.; Oh, U. Histamine-induced Ca2+ influx via the PLA2/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci. Lett. 2004, 361, 159–162. [Google Scholar] [CrossRef]
- Shim, W.-S.; Tak, M.-H.; Lee, M.-H.; Kim, M.; Koo, J.-Y.; Lee, C.-H.; Oh, U. TRPV1 Mediates Histamine-Induced Itching via the Activation of Phospholipase A2 and 12-Lipoxygenase. J. Neurosci. 2007, 27, 2331–2337. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmidt, R.; Bickel, A.; Handwerker, H.O.; Torebjörk, H.E. Specific C-Receptors for Itch in Human Skin. J. Neurosci. 1997, 17, 8003–8008. [Google Scholar] [CrossRef]
- Strakhova, M.I.; Nikkel, A.L.; Manelli, A.M.; Hsieh, G.C.; Esbenshade, T.A.; Brioni, J.D.; Bitner, R.S. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009, 1250, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.-Y.; Kang, C.-J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.-G.; Kim, D.; et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, K.; Kottke, T.; Tanrikulu, Y.; Proschak, E.; Weizel, L.; Schneider, E.; Seifert, R.; Schneider, G.; Stark, H. 2,4-Diaminopyrimidines as histamine H4 receptor ligands—Scaffold optimization and pharmacological characterization. Bioorganic Med. Chem. 2009, 17, 7186–7196. [Google Scholar] [CrossRef]
- Stark, H. Histamine H4 Receptor: A Novel Drug Target in Immunoregulation and Inflammation; Versita-deGruyter: London, UK, 2013. [Google Scholar] [CrossRef]
- Kuraishi, Y.; Nagasawa, T.; Hayashi, K.; Satoh, M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 1995, 275, 229–233. [Google Scholar] [CrossRef]
- Inagaki, N.; Nagao, M.; Igeta, K.; Kawasaki, H.; Kim, J.F.; Nagai, H. Scratching Behavior in Various Strains of Mice. Ski. Pharmacol. Physiol. 2001, 14, 87–96. [Google Scholar] [CrossRef]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef] [Green Version]
- Varga, A.; Németh, J.; Szabó, Á.; McDougall, J.; Zhang, C.; Elekes, K.; Pintér, E.; Szolcsányi, J.; Helyes, Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci. Lett. 2005, 385, 137–142. [Google Scholar] [CrossRef]
- Bäumer, W.; Roßbach, K. Histamine as an immunomodulator. J. Dtsch. Dermatol. Ges. 2010, 8, 495–504. [Google Scholar] [CrossRef]
- Cowden, J.M.; Riley, J.P.; Ma, J.Y.; Thurmond, R.L.; Dunford, P.J. Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respir. Res. 2010, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dunford, P.J.; Williams, K.N.; Desai, P.J.; Karlsson, L.; McQueen, D.; Thurmond, R. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007, 119, 176–183. [Google Scholar] [CrossRef]
- Kollmeier, A.; Francke, K.; Chen, B.; Dunford, P.J.; Greenspan, A.J.; Xia, Y.; Xu, X.L.; Zhou, B.; Thurmond, R.L. The Histamine H4 Receptor Antagonist, JNJ 39758979, Is Effective in Reducing Histamine-Induced Pruritus in a Randomized Clinical Study in Healthy Subjects. J. Pharmacol. Exp. Ther. 2014, 350, 181–187. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Hirasawa, N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. Allergy 2012, 67, 1014–1022. [Google Scholar] [CrossRef]
- Roã Bach, K.; Stark, H.; Sander, K.; Leurs, R.; Kietzmann, M.; Bäumer, W. The histamine H4 receptor as a new target for treatment of canine inflammatory skin diseases. Vet. Dermatol. 2009, 20, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, T.; Ganchingco, J.R.; Mishra, S.K.; Olivry, T.; Rzagalinski, I.; Volmer, D.A.; Bäumer, W. Janus kinase inhibitors display broad anti-itch properties: A possible link through the TRPV1 receptor. J. Allergy Clin. Immunol. 2017, 140, 306–309.e3. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.D.; van Rijn, R.M.; Ling, P.; Bakker, R.A.; Thurmond, R.L.; Leurs, R. Evaluation of Histamine H1-, H2-, and H3-Receptor Ligands at the Human Histamine H4 Receptor: Identification of 4-Methylhistamine as the First Potent and Selective H4 Receptor Agonist. J. Pharmacol. Exp. Ther. 2005, 314, 1310–1321. [Google Scholar] [CrossRef] [Green Version]
- Sugata, Y.; Okano, M.; Fujiwara, T.; Matsumoto, R.; Hattori, H.; Yamamoto, M.; Nishibori, M.; Nishizaki, K. Histamine H4 receptor agonists have more activities than H4 agonism in antigen-specific human T-cell responses. Immunology 2007, 121, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Roberson, D.P.; Gudes, S.; Sprague, J.M.; Patoski, H.A.W.; Robson, V.K.; Blasl, F.; Duan, B.; Oh, S.B.; Bean, B.P.; Ma, Q.; et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 2013, 16, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X. Targeting TRP ion channels for itch relief. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 388, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Schwendinger-Schreck, J.; Wilson, S.R.; Bautista, D.M. Interactions between Keratinocytes and Somatosensory Neurons in Itch. In Pharmacology of Itch. Handbook of Experimental Pharmacology; Cowan, A.Y.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 226, pp. 177–190. [Google Scholar] [CrossRef]
- Schaper, K.; Rossbach, K.; Köther, B.; Stark, H.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacol. Res. 2016, 113, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ru, F.; Sun, H.; Jurcakova, D.; Herbstsomer, R.A.; Meixong, J.; Dong, X.; Undem, B.J. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J. Physiol. 2017, 595, 3651–3666. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.-H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-Dependent Pruritus in IL-13–Induced Chronic Atopic Dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenezer, A.J.; Arunachalam, P.; Elden, B.T. H4R activation utilizes distinct signaling pathways for the production of RANTES and IL-13 in human mast cells. J. Recept. Signal Transduct. Res. 2016, 37, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.; Peier, A.M.; Bandell, M.; Hwang, S.W.; Huynh, T.; Olney, N.; Jegla, T.; Patapoutian, A. A Role of TRPA1 in Mechanical Hyperalgesia is Revealed by Pharmacological Inhibition. Mol. Pain 2007, 3, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gschwandtner, M.; Koether, B.; Werfel, T.; Stark, H.; Gutzmer, R. Profiling of histamine H4receptor agonists in native human monocytes. Br. J. Pharmacol. 2013, 170, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flock, T.; Hauser, A.; Lund, N.; Gloriam, D.; Balaji, S.; Babu, M.M. Selectivity determinants of GPCR–G-protein binding. Nature 2017, 545, 317–322. [Google Scholar] [CrossRef]
- Reher, T.M.; Neumann, D.; Buschauer, A.; Seifert, R. Incomplete activation of human eosinophils via the histamine H4-receptor: Evidence for ligand-specific receptor conformations. Biochem. Pharmacol. 2012, 84, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Schneider, E.; Dove, S.; Brunskole, I.; Neumann, D.; Strasser, A.; Buschauer, A. Paradoxical Stimulatory Effects of the “Standard” Histamine H4-Receptor Antagonist JNJ7777120: the H4 Receptor Joins the Club of 7 Transmembrane Domain Receptors Exhibiting Functional Selectivity. Mol. Pharmacol. 2011, 79, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Beck, J.A.; Lloyd, S.; Hafezparast, M.; Lennon-Pierce, M.; Eppig, J.T.; Festing, M.F.; Fisher, E.M. Genealogies of mouse inbred strains. Nat. Genet. 2000, 24, 23–25. [Google Scholar] [CrossRef]
- Green, A.D.; Young, K.K.; Lehto, S.G.; Smith, S.; Mogil, J.S. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 2006, 124, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Playfair, J.H.L. Strain differences in the immune response of mice: I. The neonatal response to sheep red cells. Immunology 1968, 15, 815–826. [Google Scholar] [PubMed]
- Chen, Y.; Fang, Q.; Wang, Z.; Zhang, J.Y.; MacLeod, A.S.; Hall, R.P.; Liedtke, W.B. Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminergic Itch. J. Biol. Chem. 2016, 291, 10252–10262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Tomono, A.; Suwa, E.; Ueno, K. Sex-related differences in SLIGRL-induced pruritus in mice. Life Sci. 2014, 94, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: Wheathampstead, UK, 1959. [Google Scholar]
- Akopian, A.N.; Ruparel, N.B.; Jeske, N.A.; Hargreaves, K.M. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J. Physiol. 2007, 583 Pt 1, 175–193. [Google Scholar] [CrossRef]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.; Reeh, P.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch 2014, 466, 2229–2241. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuoka, T.; Kudo, M.; Yamashita, Y.; Yoshida, J.; Imaizumi, N.; Muramatsu, I.; Nishio, M.; Ishibashi, T. TRPA1 Channels Modify TRPV1-Mediated Current Responses in Dorsal Root Ganglion Neurons. Front. Physiol. 2017, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Ruparel, N.B.; Patwardhan, A.M.; Akopian, A.N.; Hargreaves, K.M. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 2008, 135, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilzopolski, J.; Kietzmann, M.; Mishra, S.K.; Stark, H.; Bäumer, W.; Rossbach, K. TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch. Biomolecules 2021, 11, 1166. https://doi.org/10.3390/biom11081166
Wilzopolski J, Kietzmann M, Mishra SK, Stark H, Bäumer W, Rossbach K. TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch. Biomolecules. 2021; 11(8):1166. https://doi.org/10.3390/biom11081166
Chicago/Turabian StyleWilzopolski, Jenny, Manfred Kietzmann, Santosh K. Mishra, Holger Stark, Wolfgang Bäumer, and Kristine Rossbach. 2021. "TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch" Biomolecules 11, no. 8: 1166. https://doi.org/10.3390/biom11081166
APA StyleWilzopolski, J., Kietzmann, M., Mishra, S. K., Stark, H., Bäumer, W., & Rossbach, K. (2021). TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch. Biomolecules, 11(8), 1166. https://doi.org/10.3390/biom11081166