Clotrimazole Fluidizes Phospholipid Membranes and Localizes at the Hydrophobic Part near the Polar Part of the Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Differential Scanning Calorimetry
2.4. 1H NOESY MAS-NMR
2.5. Molecular Dynamics Simulations
3. Results
3.1. Differential Scanning Calorimetry Studies of the Interaction of Clotrimazole with DMPC Membranes
3.2. 1H-NMR and 1H NOESY MAS-NMR Results Indicated That Clotrimazole Is Located near the Water–Lipid Interface and Located in the Upper Part of the Hydrophobic Bilayer
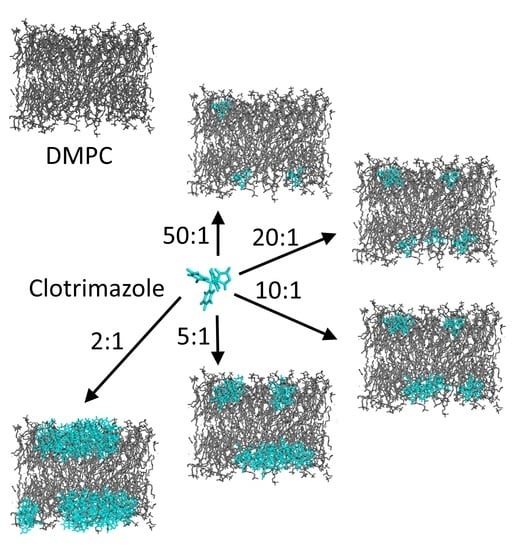
3.3. Molecular Dynamics Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, D.; Hebecker, B.; Moyes, D.L.; Miramon, P.; Jablonowski, N.; Wisgott, S.; Allert, S.; Naglik, J.R.; Hube, B. Clotrimazole dampens vaginal inflammation and neutrophil infiltration in response to Candida albicans infection. Antimicrob. Agents Chemother. 2013, 57, 5178–5180. [Google Scholar] [CrossRef] [Green Version]
- Czerninski, R.; Pikovsky, A.; Gati, I.; Friedman, M.; Steinberg, D. Comparison of the efficacy of a novel sustained release clotrimazole varnish and clotrimazole troches for the treatment of oral candidiasis. Clin. Oral Investig. 2015, 19, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.G.; Dodman, B.; Williamson, D.M.; Brown, G.J.; Bowen, R.G. Comparison of terbinafine and clotrimazole in treating tinea pedis. BMJ 1993, 307, 645–647. [Google Scholar] [CrossRef] [Green Version]
- Hitchcock, C.A.; Dickinson, K.; Brown, S.B.; Evans, E.G.; Adams, D.J. Interaction of azole antifungal antibiotics with cytochrome P-450-dependent 14 alpha-sterol demethylase purified from Candida albicans. Biochem. J. 1990, 266, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bartolommei, G.; Tadini-Buoninsegni, F.; Hua, S.; Moncelli, M.R.; Inesi, G.; Guidelli, R. Clotrimazole inhibits the Ca2+-ATPase (SERCA) by interfering with Ca2+ binding and favoring the E2 conformation. J. Biol. Chem. 2006, 281, 9547–9551. [Google Scholar] [CrossRef] [Green Version]
- Witzke, A.; Lindner, K.; Munson, K.; Apell, H.J. Inhibition of the gastric H,K-ATPase by clotrimazole. Biochemistry 2010, 49, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Bartolommei, G.; Devaux, N.; Tadini-Buoninsegni, F.; Moncelli, M.; Apell, H.J. Effect of clotrimazole on the pump cycle of the Na,K-ATPase. Biophys. J. 2008, 95, 1813–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, Y.M.; Connor, B.L. Comparison of clotrimazole cream, Whitfield’s ointment and Nystatin ointment for the topical treatment of ringworm infections, pityriasis versicolor, erythrasma and candidiasis. Br. J. Dermatol. 1973, 89, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, P.R.; Brogden, R.N.; Pinder, R.M.; Speight, T.M.; Avery, G.S. Clotrimazole: A review of its antifungal activity and therapeutic efficacy. Drugs 1975, 9, 424–447. [Google Scholar] [CrossRef]
- Lee, C.M.; Maibach, H.I. Deep percutaneous penetration into muscles and joints. J. Pharm. Sci. 2006, 95, 1405–1413. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies. Molecules 2019, 24, 3139. [Google Scholar] [CrossRef] [Green Version]
- Manca, M.L.; Usach, I.; Peris, J.E.; Ibba, A.; Orru, G.; Valenti, D.; Escribano-Ferrer, E.; Gomez-Fernandez, J.C.; Aranda, F.J.; Fadda, A.M.; et al. Optimization of Innovative Three-Dimensionally-Structured Hybrid Vesicles to Improve the Cutaneous Delivery of Clotrimazole for the Treatment of Topical Candidiasis. Pharmaceutics 2019, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, C.J.F.; Van Gent, C.M.; Pries, C. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D. The interaction of small molecules with phospholipid membranes studied by 1H NOESY NMR under magic-angle spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic. Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Hess, B.; van der Spoel, D.; Lindahl, E. GROMACS User Manual Version 5.0.7. 2015. Available online: https://ftp.gromacs.org/pub/manual/manual-5.0.7.pdf (accessed on 1 March 2021).
- Oostenbrink, C.; Villa, A.; Mark, A.E.; van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrink, C.; Soares, T.A.; van der Vegt, N.F.; van Gunsteren, W.F. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukol, A. Lipid Models for United-Atom Molecular Dynamics Simulations of Proteins. J. Chem. Theory Comput. 2009, 5, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Poger, D.; Van Gunsteren, W.F.; Mark, A.E. A new force field for simulating phosphatidylcholine bilayers. J. Comput. Chem. 2010, 31, 1117–1125. [Google Scholar] [CrossRef]
- Poger, D.; Mark, A.E. On the Validation of Molecular Dynamics Simulations of Saturated and cis-Monounsaturated Phosphatidylcholine Lipid Bilayers: A Comparison with Experiment. J. Chem. Theory Comput. 2010, 6, 325–336. [Google Scholar] [CrossRef]
- Koziara, K.B.; Stroet, M.; Malde, A.K.; Mark, A.E. Testing and validation of the Automated Topology Builder (ATB) version 2.0: Prediction of hydration free enthalpies. J. Comput.-Aided Mol. Des. 2014, 28, 221–233. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Ausili, A.; Rodriguez-Gonzalez, I.; Torrecillas, A.; Teruel, J.A.; Gomez-Fernandez, J.C. Diethylstilbestrol Modifies the Structure of Model Membranes and Is Localized Close to the First Carbons of the Fatty Acyl Chains. Biomolecules 2021, 11, 220. [Google Scholar] [CrossRef]
- Ausili, A.; Gomez-Murcia, V.; Candel, A.M.; Beltran, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gomez-Fernandez, J.C. A comparison of the location in membranes of curcumin and curcumin-derived bivalent compounds with potential neuroprotective capacity for Alzheimer’s disease. Colloids Surf. B Biointerfaces 2020, 199, 111525. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Gomez-Fernandez, J.C. A differential scanning calorimetry study of the interaction of free fatty acids with phospholipid membranes. Chem. Phys. Lipids 1987, 45, 75–91. [Google Scholar] [CrossRef]
- Huster, D.; Arnold, K.; Gawrisch, K. Investigation of Lipid Organization in Biological Membranes by Two-Dimensional Nuclear Overhauser Enhancement Spectroscopy. J. Phys. Chem. B 1999, 103, 243–251. [Google Scholar] [CrossRef]
- Ausili, A.; Clemente, J.; Pons-Belda, O.D.; de Godos, A.; Corbalan-Garcia, S.; Torrecillas, A.; Teruel, J.A.; Gomez-Fernandez, J.C. Interaction of Vitamin K1 and Vitamin K2 with Dimyristoylphosphatidylcholine and Their Location in the Membrane. Langmuir 2020, 36, 1062–1073. [Google Scholar] [CrossRef]
- Ortiz, A.; Aranda, F.J. The influence of vitamin K1 on the structure and phase behaviour of model membrane systems. Biochim. Biophys. Acta 1999, 1418, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Perez-Lara, A.; Ausili, A.; Aranda, F.J.; de Godos, A.; Torrecillas, A.; Corbalan-Garcia, S.; Gomez-Fernandez, J.C. Curcumin disorders 1,2-dipalmitoyl-sn-glycero-3-phosphocholine membranes and favors the formation of nonlamellar structures by 1,2-dielaidoyl-sn-glycero-3-phosphoethanolamine. J. Phys. Chem. B 2010, 114, 9778–9786. [Google Scholar] [CrossRef]
- Sanchez-Migallon, M.P.; Aranda, F.J.; Gomez-Fernandez, J.C. Interaction between alpha-tocopherol and heteroacid phosphatidylcholines with different amounts of unsaturation. Biochim. Biophys. Acta 1996, 1279, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Scheidt, H.A.; Feller, S.E.; Metso, J.; Badeau, R.M.; Tikkanen, M.J.; Wahala, K.; Jauhiainen, M.; Huster, D. The orientation and dynamics of estradiol and estradiol oleate in lipid membranes and HDL disc models. Biophys. J. 2014, 107, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Windshügel, B.; Jyrkkärinne, J.; Poso, A.; Honkakoski, P.; Sippl, W. Molecular dynamics simulations of the human CAR ligand-binding domain: Deciphering the molecular basis for constitutive activity. J. Mol. Model. 2005, 11, 69–79. [Google Scholar] [CrossRef]
- Chao, W.-C.; Lu, J.-F.; Wang, J.-S.; Yang, H.-C.; Chen, H.-H.; Lan, Y.-K.; Yu, Y.-C.; Chou, P.-T.; Wang, L.-H. Probing the Interaction between Prostacyclin Synthase and Prostaglandin H2 Analogues or Inhibitors via a Combination of Resonance Raman Spectroscopy and Molecular Dynamics Simulation Approaches. J. Am. Chem. Soc. 2011, 133, 18870–18879. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Baaden, M.; Wang, J.; Liang, Z. A cooperative mechanism of clotrimazoles in P450 revealed by the dissociation picture of clotrimazole from P450. J. Chem. Inf. Model. 2014, 54, 1218–1225. [Google Scholar] [CrossRef] [PubMed]








| [Clotrimazole]/[DMPC] | Molar Ratio DMPC:Clotrimazole | ΔH (kcal/mol) |
|---|---|---|
| 0 | 1:0 | 6.626 ± 0.040 |
| 0.02 | 50:1 | 6.548 ± 0.036 |
| 0.05 | 20:1 | 6.386 ± 0.284 |
| 0.1 | 10:1 | 6.316 ± 0.182 |
| 0.2 | 5:1 | 6.342 ± 0.090 |
| 0.5 | 2:1 | 6.348 ± 0.411 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ausili, A.; Yakymenko, I.; Teruel, J.A.; Gómez-Fernández, J.C. Clotrimazole Fluidizes Phospholipid Membranes and Localizes at the Hydrophobic Part near the Polar Part of the Membrane. Biomolecules 2021, 11, 1304. https://doi.org/10.3390/biom11091304
Ausili A, Yakymenko I, Teruel JA, Gómez-Fernández JC. Clotrimazole Fluidizes Phospholipid Membranes and Localizes at the Hydrophobic Part near the Polar Part of the Membrane. Biomolecules. 2021; 11(9):1304. https://doi.org/10.3390/biom11091304
Chicago/Turabian StyleAusili, Alessio, Illya Yakymenko, José A. Teruel, and Juan C. Gómez-Fernández. 2021. "Clotrimazole Fluidizes Phospholipid Membranes and Localizes at the Hydrophobic Part near the Polar Part of the Membrane" Biomolecules 11, no. 9: 1304. https://doi.org/10.3390/biom11091304
APA StyleAusili, A., Yakymenko, I., Teruel, J. A., & Gómez-Fernández, J. C. (2021). Clotrimazole Fluidizes Phospholipid Membranes and Localizes at the Hydrophobic Part near the Polar Part of the Membrane. Biomolecules, 11(9), 1304. https://doi.org/10.3390/biom11091304