Mediterranean Diet Food Components as Possible Adjuvant Therapies to Counteract Breast and Prostate Cancer Progression to Bone Metastasis
Abstract
:1. Introduction
2. Methods
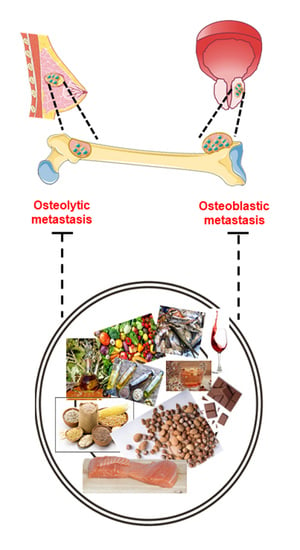
3. Bone Metastasis: A Multi-Step Process
3.1. Epithelial-Mesenchymal Transition: A Process That Regulates Invasiveness
3.2. Skeleton Colonization: Alteration of Physiological Bone Remodeling
3.2.1. Osteolytic Bone Metastasis
3.2.2. Osteoblastic Bone Metastasis
4. Nutrients and Bone Metastasis
4.1. Nutrients in the Epithelial-Mesenchymal Plasticity
4.2. Nutrients in the Osteolytic Bone Metastasis
4.3. Nutrients in Osteoblastic Bone Metastasis
4.4. Nutrients with an Assessed Anti-Bone Metastatic Role although Not Specifically Related to Breast/Prostate Cancer or to the Mechanisms Described Above
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hagaman, D.E.; Damasco, J.A.; Perez, J.V.D.; Rojo, R.D.; Melancon, M.P. Recent Advances in Nanomedicine for the Diagnosis and Treatment of Prostate Cancer Bone Metastasis. Molecules 2021, 26, 384. [Google Scholar] [CrossRef] [PubMed]
- Galasko, C.S. Monitoring of bone metastases. Schweiz. Med. Wochenschr. 1981, 111, 1873–1875. [Google Scholar] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoni, M.; Conti, A.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Guida, F.; Fontana, A.; Berruti, A.; Berardi, R.; et al. Bone metastases in patients with metastatic renal cell carcinoma: Are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 2015, 34, 10. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.; Badia, X.; Chow, E.; Lipton, A.; Wardley, A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support. Care Cancer 2008, 16, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M.J. Nutrition and cancer: Prevention and survival. Br. J. Nutr. 2019, 122, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Di Maso, M.; Maso, L.D.; Augustin, L.S.A.; Puppo, A.; Falcini, F.; Stocco, C.; Mattioli, V.; Serraino, D.; Polesel, J. Adherence to the Mediterranean Diet and Mortality after Breast Cancer. Nutrients 2020, 12, 3649. [Google Scholar] [CrossRef]
- Tang, X.; Shi, L.; Xie, N.; Liu, Z.; Qian, M.; Meng, F.; Xu, Q.; Zhou, M.; Cao, X.; Zhu, W.-G.; et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat. Commun. 2017, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liss, M.A.; Schlicht, M.; Kahler, A.; Fitzgerald, R.; Thomassi, T.; Degueme, A.; Hessner, M.; Datta, M.W. Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteom. 2010, 7, 245–252. [Google Scholar]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguley, B.J.; Skinner, T.L.; Jenkins, D.G.; Wright, O.R.L. Mediterranean-style dietary pattern improves cancer-related fatigue and quality of life in men with prostate cancer treated with androgen deprivation therapy: A pilot randomised control trial. Clin. Nutr. 2021, 40, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Ozaki, Y.; Imazeki, H.; Hayashi, H.; Masuda, J.; Ozawa, H.; Ogiwara, Y. Targeting oncoimmune drivers of cancer metastasis. Cancers 2021, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [Green Version]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Signaling via Shc family adapter proteins. Oncogene 2001, 20, 6322–6330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi Roshan, M.; Soltani, A.; Soleimani, A.; Rezaie Kahkhaie, K.; Afshari, A.R.; Soukhtanloo, M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie 2019, 165, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jin, M.; Li, K.; Liu, H.; Yang, X.; Zhang, X.; Zhang, B.; Gong, A.; Bie, Q. TRAF6 Promotes Gastric Cancer Cell Self-Renewal, Proliferation, and Migration. Stem Cells Int. 2020, 2020, 3296192. [Google Scholar] [CrossRef]
- Shome, R.; Ghosh, S.S. Tweaking EMT and MDR dynamics to constrain triple-negative breast cancer invasiveness by EGFR and Wnt/β-catenin signaling regulation. Cell. Oncol. 2021, 44, 405–422. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Y.; Wang, Y.; Chen, Y.; Li, M.; Jiang, Y. Hepatocyte growth factor increases the invasive potential of PC-3 human prostate cancer cells via an ERK/MAPK and Zeb-1 signaling pathway. Oncol. Lett. 2016, 11, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of Latent Transforming Growth Factor-β (TGF-β)-binding Protein-1 by Osteoclasts. J. Biol. Chem. 2002, 277, 21352–21360. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Zarrer, J.; Haider, M.-T.; Smit, D.J.; Taipaleenmäki, H. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeton, Z.S.; Lie, W.-R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia 2015, 17, 849–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroni, P.; Bendinelli, P. Bone, a Secondary Growth Site of Breast and Prostate Carcinomas: Role of Osteocytes. Cancers 2020, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P. Megakaryocytes in Bone Metastasis: Protection or Progression? Cells 2019, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, C.; Roudier, M.P.; Dowell, A.; True, L.D.; Ketchanji, M.; Welty, C.; Corey, E.; Lange, P.H.; Higano, C.S.; Vessella, R.L. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: Results from the University of Washington Rapid Autopsy Series. J. Bone Miner. Res. 2013, 28, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.S.; Gmyrek, G.A.; Inra, J.; Scherr, D.S.; Vaughan, E.D.; Nanus, D.M.; Kattan, M.W.; Gerald, W.L.; Vande Woude, G.F. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002, 60, 1113–1117. [Google Scholar] [CrossRef]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M. Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia. Int. J. Mol. Sci. 2016, 17, 706. [Google Scholar] [CrossRef] [Green Version]
- Previdi, S.; Maroni, P.; Matteucci, E.; Broggini, M.; Bendinelli, P.; Desiderio, M.A. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and β-catenin/Wnt pathways. Eur. J. Cancer 2010, 46, 1679–1691. [Google Scholar] [CrossRef]
- Maroni, P.; Puglisi, R.; Mattia, G.; Carè, A.; Matteucci, E.; Bendinelli, P.; Desiderio, M.A. In bone metastasis miR-34a-5p absence inversely correlates with Met expression, while Met oncogene is unaffected by miR-34a-5p in non-metastatic and metastatic breast carcinomas. Carcinogenesis 2017, 38, 492–503. [Google Scholar] [CrossRef]
- Bendinelli, P.; Maroni, P.; Dall’Olio, V.; Matteucci, E.; Desiderio, M.A. Bone Metastasis Phenotype and Growth Undergo Regulation by Micro-Environment Stimuli: Efficacy of Early Therapy with HGF or TGFβ1-Type I Receptor Blockade. Int. J. Mol. Sci. 2019, 20, 2520. [Google Scholar] [CrossRef] [Green Version]
- Guise, T.A.; Yin, J.J.; Taylor, S.D.; Kumagai, Y.; Dallas, M.; Boyce, B.F.; Yoneda, T.; Mundy, G.R. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 1996, 98, 1544–1549. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Ferraretto, A.; Lombardi, G. Interleukin 11 (IL-11): Role(s) in Breast Cancer Bone Metastases. Biomedicines 2021, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Guise, T.A.; Yin, J.J.; Elliott, J.; Horwood, N.J.; Martin, T.J.; Gillespie, M.T. Breast Cancer Cells Interact with Osteoblasts to Support Osteoclast Formation1. Endocrinology 1999, 140, 4451–4458. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.A.; Oyajobi, B.O.; Grubbs, B.; Padalecki, S.S.; Munoz, S.A.; Gupta, A.; Story, B.; Zhao, M.; Mundy, G.R. The Hedgehog Signaling Molecule Gli2 Induces Parathyroid Hormone-Related Peptide Expression and Osteolysis in Metastatic Human Breast Cancer Cells. Cancer Res. 2006, 66, 7548–7553. [Google Scholar] [CrossRef] [Green Version]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-Derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Bu, G.; Lu, W.; Liu, C.; Selander, K.; Yoneda, T.; Hall, C.; Keller, E.T.; Li, Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int. J. Cancer 2008, 123, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Tarragona, M.; Pavlovic, M.; Arnal-Estapé, A.; Urosevic, J.; Morales, M.; Guiu, M.; Planet, E.; González-Suárez, E.; Gomis, R.R. Identification of NOG as a Specific Breast Cancer Bone Metastasis-supporting Gene. J. Biol. Chem. 2012, 287, 21346–21355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.J.; Mohammad, K.S.; Kakonen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, K.S.; Guise, T.A. Mechanisms of Osteoblastic Metastases: Role of Endothelin-1. Clin. Orthop. Relat. Res. 2003, 415, S67–S74. [Google Scholar] [CrossRef]
- Hall, C.L.; Bafico, A.; Dai, J.; Aaronson, S.A.; Keller, E.T. Prostate Cancer Cells Promote Osteoblastic Bone Metastases through Wnts. Cancer Res. 2005, 65, 7554–7560. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, J.; Bao, M.; Zeng, K.; Fu, S.; Wang, C.; Ye, L. Wnt signaling in bone metastasis: Mechanisms and therapeutic opportunities. Life Sci. 2018, 208, 33–45. [Google Scholar] [CrossRef]
- Zheng, D.; Decker, K.F.; Zhou, T.; Chen, J.; Qi, Z.; Jacobs, K.; Weilbaecher, K.N.; Corey, E.; Long, F.; Jia, L. Role of WNT7B-induced Noncanonical Pathway in Advanced Prostate Cancer. Mol. Cancer Res. 2013, 11, 482–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-C.; Lee, Y.-C.; Yu, G.; Cheng, C.-J.; Zhou, X.; Chu, K.; Murshed, M.; Le, N.-T.; Baseler, L.; Abe, J.; et al. Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev. Cell 2017, 41, 467–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucci, N.; Teti, A. Osteomimicry: How the Seed Grows in the Soil. Calcif. Tissue Int. 2018, 102, 131–140. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Zheng, L.; Choe, C.; Lindgren, B.; Jensen, E.D.; Westendorf, J.J.; Cheng, L.; Huang, H. FOXO1 Inhibits Runx2 Transcriptional Activity and Prostate Cancer Cell Migration and Invasion. Cancer Res. 2011, 71, 3257–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.L.; Zhang, H.; Baile, S.; Ljungman, M.; Kuhstoss, S.; Keller, E.T. p21 CIP-1/WAF-1 Induction Is Required to Inhibit Prostate Cancer Growth Elicited by Deficient Expression of the Wnt Inhibitor Dickkopf-1. Cancer Res. 2010, 70, 9916–9926. [Google Scholar] [CrossRef] [Green Version]
- Secondini, C.; Wetterwald, A.; Schwaninger, R.; Thalmann, G.N.; Cecchini, M.G. The Role of the BMP Signaling Antagonist Noggin in the Development of Prostate Cancer Osteolytic Bone Metastasis. PLoS ONE 2011, 6, e16078. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekita, A.; Matsugaki, A.; Nakano, T. Disruption of collagen/apatite alignment impairs bone mechanical function in osteoblastic metastasis induced by prostate cancer. Bone 2017, 97, 83–93. [Google Scholar] [CrossRef]
- Sun, C.; Hu, Y.; Guo, T.; Wang, H.; Zhang, X.; He, W.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef] [Green Version]
- Vergara, D.; Valente, C.M.; Tinelli, A.; Siciliano, C.; Lorusso, V.; Acierno, R.; Giovinazzo, G.; Santino, A.; Storelli, C.; Maffia, M. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011, 310, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Go, R.-E.; Hwang, K.-A.; Choi, K.-C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.; Wu, J.M. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chong, T.; Wang, Z.; Chen, H.; Li, H.; Cao, J.; Zhang, P.; Li, H. A novel anti-cancer effect of resveratrol: Reversal of epithelial-mesenchymal transition in prostate cancer cells. Mol. Med. Rep. 2014, 10, 1717–1724. [Google Scholar] [CrossRef] [Green Version]
- Chu, Q.; Ling, M.-T.; Feng, H.; Cheung, H.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. A novel anticancer effect of garlic derivatives: Inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006, 27, 2180–2189. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.-Y.; Kim, J.-I.; Yoon, M.K.; Kwon, T.K.; Lee, S.J.; Choi, Y.-W.; Kang, H.S.; Yoo, Y.H.; Choi, Y.H. Anti-invasive activity of diallyl disulfide through tightening of tight junctions and inhibition of matrix metalloproteinase activities in LNCaP prostate cancer cells. Toxicol. In Vitro 2010, 24, 1569–1576. [Google Scholar] [CrossRef]
- Huang, J.; Yang, B.; Xiang, T.; Peng, W.; Qiu, Z.; Wan, J.; Zhang, L.; Li, H.; Li, H.; Ren, G. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the β-catenin signaling pathway. Mol. Nutr. Food Res. 2015, 59, 1063–1075. [Google Scholar] [CrossRef]
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive secoiridoids and semisynthetic bioisostere analogues for the control of metastatic breast cancer. Bioorg. Med. Chem. 2013, 21, 2117–2127. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive Phenolics as c-Met Inhibitors: (-)-Oleocanthal Attenuates Cell Proliferation, Invasiveness, and Tumor Growth in Breast Cancer Models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-A.; Choi, K.-C.; Hwang, K.-A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Onco Targets Ther. 2017, 10, 4809–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.-N.; Jun, W.; Choue, R.; Lee, J. I3C and ICZ inhibit migration by suppressing the EMT process and FAK expression in breast cancer cells. Mol. Med. Rep. 2013, 7, 384–388. [Google Scholar] [CrossRef]
- Arzi, L.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R.; Jafarzadeh, N. A Comparative Study on Anti-Invasion, Antimigration, and Antiadhesion Effects of the Bioactive Carotenoids of Saffron on 4T1 Breast Cancer Cells through Their Effects on Wnt/β-Catenin Pathway Genes. DNA Cell Biol. 2018, 37, 697–707. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wu, D.; Fan, J.; Li, X.; Wu, K.; Wang, X.; He, D. A novel anti-cancer effect of genistein: Reversal of epithelial mesenchymal transition in prostate cancer cells 1. Acta Pharmacol. Sin. 2008, 29, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Kumi-Diaka, J.K.; Hassanhi, M.; Merchant, K.; Horman, V. Influence of Genistein Isoflavone on Matrix Metalloproteinase-2 Expression in Prostate Cancer Cells. J. Med. Food 2006, 9, 491–497. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar]
- Li, Y.; Che, M.; Bhagat, S.; Ellis, K.-L.; Kucuk, O.; Doerge, D.R.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Regulation of Gene Expression and Inhibition of Experimental Prostate Cancer Bone Metastasis by Dietary Genistein. Neoplasia 2004, 6, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.J.; Barnett, P.; Smith, B.; Arnold, R.S.; Hudson, T.; Kundu, K.; Murthy, N.; Odero-Marah, V.A. Muscadine grape skin extract reverts snail-mediated epithelial mesenchymal transition via superoxide species in human prostate cancer cells. BMC Complement. Altern. Med. 2014, 14, 97. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.J.; Smith, B.A.; Smith, B.N.; Loyd, Q.; Nagappan, P.; McKeithen, D.; Wilder, C.L.; Platt, M.O.; Hudson, T.; Odero-Marah, V.A. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis 2015, 36, 1019–1027. [Google Scholar] [CrossRef]
- Wu, K.; Zeng, J.; Zhu, G.; Zhang, L.; Zhang, D.; Li, L.; Fan, J.; Wang, X.; He, D. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol. Sin. 2009, 30, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Zeng, J.; Li, L.; Fan, J.; Zhang, D.; Xue, Y.; Zhu, G.; Yang, L.; Wang, X.; He, D. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol. Rep. 2010, 23, 1545–1552. [Google Scholar] [PubMed]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Pichardo, L.; Martínez-Montemayor, M.M.; Martínez, J.E.; Wall, K.M.; Cubano, L.A.; Dharmawardhane, S. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin. Exp. Metastasis 2009, 26, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.E.; Frye, J.B.; Lukefahr, A.L.; Timmermann, B.N.; Mohammad, K.S.; Guise, T.A.; Funk, J.L. Curcuminoids Block TGF-β Signaling in Human Breast Cancer Cells and Limit Osteolysis in a Murine Model of Breast Cancer Bone Metastasis. J. Nat. Prod. 2013, 76, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunihiro, A.G.; Brickey, J.A.; Frye, J.B.; Luis, P.B.; Schneider, C.; Funk, J.L. Curcumin, but not curcumin-glucuronide, inhibits Smad signaling in TGFβ-dependent bone metastatic breast cancer cells and is enriched in bone compared to other tissues. J. Nutr. Biochem. 2019, 63, 150–156. [Google Scholar] [CrossRef]
- Wright, L.; Frye, J.; Gorti, B.; Timmermann, B.; Funk, J. Bioactivity of Turmeric-derived Curcuminoids and Related Metabolites in Breast Cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef] [Green Version]
- Bharti, A.C.; Takada, Y.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Receptor Activator of NF-κB Ligand-Induced NF-κB Activation in Osteoclast Precursors and Suppresses Osteoclastogenesis. J. Immunol. 2004, 172, 5940–5947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, K.-W.; Ko, C.-H.; Yue, G.G.-L.; Lee, J.K.-M.; Li, K.-K.; Lee, M.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. Green tea (Camellia sinensis) extract inhibits both the metastasis and osteolytic components of mammary cancer 4T1 lesions in mice. J. Nutr. Biochem. 2014, 25, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Pore, S.K.; Hahm, E.-R.; Kim, S.-H.; Singh, K.B.; Nyiranshuti, L.; Latoche, J.D.; Anderson, C.J.; Adamik, J.; Galson, D.L.; Weiss, K.R.; et al. A Novel Sulforaphane-Regulated Gene Network in Suppression of Breast Cancer–Induced Osteolytic Bone Resorption. Mol. Cancer Ther. 2020, 19, 420–431. [Google Scholar] [CrossRef]
- Mandal, C.C.; Ghosh-Choudhury, T.; Yoneda, T.; Choudhury, G.G.; Ghosh-Choudhury, N. Fish oil prevents breast cancer cell metastasis to bone. Biochem. Biophys. Res. Commun. 2010, 402, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Alhambra, D.; Servitja, S.; Javaid, M.K.; Garrigós, L.; Arden, N.K.; Cooper, C.; Albanell, J.; Tusquets, I.; Diez-Perez, A.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-related bone loss: The B-ABLE prospective cohort study. Breast Cancer Res. Treat. 2012, 133, 1159–1167. [Google Scholar] [CrossRef]
- Hutchins-Wiese, H.L.; Picho, K.; Watkins, B.A.; Li, Y.; Tannenbaum, S.; Claffey, K.; Kenny, A.M. High-Dose Eicosapentaenoic Acid and Docosahexaenoic Acid Supplementation Reduces Bone Resorption in Postmenopausal Breast Cancer Survivors on Aromatase Inhibitors: A Pilot Study. Nutr. Cancer 2014, 66, 68–76. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, B.; Jin, W.J.; Kim, J.-W.; Kim, H.-H.; Ha, H.; Lee, Z.H. Trolox inhibits osteolytic bone metastasis of breast cancer through both PGE2-dependent and independent mechanisms. Biochem. Pharmacol. 2014, 91, 51–60. [Google Scholar] [CrossRef]
- Winzer, M.; Rauner, M.; Pietschmann, P. Glycitein decreases the generation of murine osteoclasts and increases apoptosis. Wien. Med. Wochenschr. 2010, 160, 446–451. [Google Scholar] [CrossRef]
- Alonso, V.; Pérez-Martínez, F.C.; Calahorra, F.J.; Esbrit, P. Phytoestrogen modulation of bone-related cytokines and its impact on cell viability in human prostate cancer cells. Life Sci. 2009, 85, 421–430. [Google Scholar] [CrossRef]
- Park, K.; Ju, W.-C.; Yeo, J.-H.; Kim, J.Y.; Seo, H.S.; Uchida, Y.; Cho, Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int. J. Mol. Med. 2014, 33, 178–184. [Google Scholar] [CrossRef]
- Ooi, L.L.; Zhou, H.; Kalak, R.; Zheng, Y.; Conigrave, A.D.; Seibel, M.J.; Dunstan, C.R. Vitamin D Deficiency Promotes Human Breast Cancer Growth in a Murine Model of Bone Metastasis. Cancer Res. 2010, 70, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Shen, J.; Si, W.; Du, C.; Chen, D.; Xu, L.; Yao, M.; Fu, P.; Fan, W. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 2017, 8, 65743–65758. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Murata, T.; Shoji, M.; Weitzmann, M.N. The flavonoid p-hydroxycinnamic acid mediates anticancer effects on MDA-MB-231 human breast cancer cells in vitro: Implications for suppression of bone metastases. Int. J. Oncol. 2015, 47, 1563–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skommer, J.; Wlodkowic, D.; Pelkonen, J. Gene-expression profiling during curcumin-induced apoptosis reveals downregulation of CXCR4. Exp. Hematol. 2007, 35, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Down-Regulates Expression of Cell Proliferation and Antiapoptotic and Metastatic Gene Products through Suppression of IκBα Kinase and Akt Activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, J.G.; Stadelman, H.L.; Roselli, C.E. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int. J. Oncol. 2009, 34, 1319–1327. [Google Scholar] [PubMed]
- Dorai, T.; Diouri, J.; O’Shea, O.; Doty, S.B. Curcumin Inhibits Prostate Cancer Bone Metastasis by Up-Regulating Bone Morphogenic Protein-7 in vivo. J. Cancer Ther. 2014, 05, 369–386. [Google Scholar] [CrossRef] [Green Version]
- Augustsson, K.; Michaud, D.S.; Rimm, E.B.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol. Biomakers Prev. 2003, 12, 64–67. [Google Scholar]
- Brown, M.D.; Hart, C.A.; Gazi, E.; Bagley, S.; Clarke, N.W. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br. J. Cancer 2006, 94, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.R.; Yang, G.; Timme, T.L.; Park, S.H.; Kadmon, D.; Ren, C.; Ji, X.; Lee, H.M.; Sehgal, I.; Anzano, M.; et al. Dietary 4-HPR suppresses the development of bone metastasis in vivo in a mouse model of prostate cancer progression. Clin. Exp. Metastasis 2000, 18, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Lin, Y.-W.; Wen, Y.-C.; Yang, Y.-C.; Hsiao, M.; Chang, J.-L.; Huang, H.-C.; Lee, W.-J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Jung, J.; Moon, A.; Kang, H.; Cho, H. Antitumor and Anti-Invasive Effect of Apigenin on Human Breast Carcinoma through Suppression of IL-6 Expression. Int. J. Mol. Sci. 2019, 20, 3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef] [Green Version]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarini, A.; Pasanisi, P.; Traina, A.; Mano, M.P.; Bonanni, B.; Panico, S.; Scipioni, C.; Galasso, R.; Paduos, A.; Simeoni, M.; et al. Lifestyle and breast cancer recurrences: The DIANA-5 trial. Tumori 2012, 98, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Porciello, G.; Montagnese, C.; Crispo, A.; Grimaldi, M.; Libra, M.; Vitale, S.; Palumbo, E.; Pica, R.; Calabrese, I.; Cubisino, S.; et al. Mediterranean diet and quality of life in women treated for breast cancer: A baseline analysis of DEDiCa multicentre trial. PLoS ONE 2020, 15, e0239803. [Google Scholar] [CrossRef]
- Maureen Sheean, P.; Robinson, P.; Bartolotta, M.B.; Joyce, C.; Adams, W.; Penckofer, S. Associations between cholecalciferol supplementation and self-reported symptoms among women with metastatic breast cancer and vitamin d deficiency: A pilot study. Oncol. Nurs. Forum 2021, 48, 352–360. [Google Scholar] [CrossRef]


| Biomolecules | Foods | References |
|---|---|---|
| resveratrol | grapes, red wine, peanuts, berries | [59,61,62,63,64,65,66,103] |
| s-allylcysteine (SAC) s-allylmercaptocysteine (SAMC) | broccoli, Brussels sprouts, cauliflowers | [67] |
| diallyl disulfide (DADS) | garlic | [68,69] |
| (-)-oleocanthal | extra virgin olive oil | [70,71] |
| kaempferol | leafy vegetables, apples, onions, broccoli, berries, tea, cabbage, endive, kale, beans, tomato, strawberries, leeks, grapes | [72,73,74] |
| indole-3-carbinol (I3C) indole[3,2-b] carbazole (ICZ) | cauliflower, cabbage, kale, garden cress, bok choy, broccoli, Brussels sprouts, mustard plants, leafy vegetables | [75] |
| crocin crocetin | saffron | [76] |
| genistein (4′,5,7-trihydroxyisoflavone) | soy | [77,78,79,80] |
| anthocyanin 3,5-diglucosides | berries, currants, grapes, tropical fruits, leafy vegetables, grains, roots, tubers | [81,82] |
| silibinin | milk thistle | [84] |
| curcumin | curry powder | [85,88,89,90,91,105,106,107,108,114,115] |
| ferulic acid | rice, wheat, oats, pineapple, grapefruit, orange, banana, berries, vegetables, flowers, leaves, beans, coffee beans, artichoke, peanut, nuts | [86] |
| quercetin | kale, tomatoes, broccoli, blueberries, apples | [87] |
| catechin epicatechin (EC) ec gallate (ECG) epigallocatechin (EGC) egc gallate (EGCG) | red wine, chocolate, tea, almonds, apples, blackberries, fava beans, hazelnuts, pistachios, plums, raspberries, strawberries | [92] |
| sulforaphane | cabbage, cauliflower, Brussels sprouts, bok choy, kale, collards, mustard greens, watercress | [93] |
| docosahexaenoic acid (DHA) eicosapentaenoic acid (EPA) | salmon, foraging fish, shellfish, tuna, walnuts, sardines, herring, mackerel, halibut | [94,95,97,109,110] |
| vitamin D | tuna, mackerel, salmon, cheese, egg yolks | [96,102] |
| trolox (vitamin E derivative) | wheat germ oil, sunflower seeds, almonds, sunflower oil, hazelnuts, peanut butter, corn oil, spinach, broccoli, soybean oil, kiwi fruit, mango, tomato, spinach | [98] |
| glycitein | soy and soy products, | [99] |
| daidzein and genistein | soy and soy products | [100,101] |
| p-hydroxycinnamic acid (HCA) | wasabi leafstalk, coffee, tea, wine, apples, berries, plums, cherries, peaches, citrus fruits, carrots, salad, cabbage, eggplant, artichoke, cereals, grapes | [104] |
| n-(4-hydroxyphenyl) retinamide (4-HPR) | synthetic retinoid | [111] |
| apigenin (API) | parsley, celery, celeriac, chamomile tea | [112,113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroni, P.; Bendinelli, P.; Fulgenzi, A.; Ferraretto, A. Mediterranean Diet Food Components as Possible Adjuvant Therapies to Counteract Breast and Prostate Cancer Progression to Bone Metastasis. Biomolecules 2021, 11, 1336. https://doi.org/10.3390/biom11091336
Maroni P, Bendinelli P, Fulgenzi A, Ferraretto A. Mediterranean Diet Food Components as Possible Adjuvant Therapies to Counteract Breast and Prostate Cancer Progression to Bone Metastasis. Biomolecules. 2021; 11(9):1336. https://doi.org/10.3390/biom11091336
Chicago/Turabian StyleMaroni, Paola, Paola Bendinelli, Alessandro Fulgenzi, and Anita Ferraretto. 2021. "Mediterranean Diet Food Components as Possible Adjuvant Therapies to Counteract Breast and Prostate Cancer Progression to Bone Metastasis" Biomolecules 11, no. 9: 1336. https://doi.org/10.3390/biom11091336
APA StyleMaroni, P., Bendinelli, P., Fulgenzi, A., & Ferraretto, A. (2021). Mediterranean Diet Food Components as Possible Adjuvant Therapies to Counteract Breast and Prostate Cancer Progression to Bone Metastasis. Biomolecules, 11(9), 1336. https://doi.org/10.3390/biom11091336