Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems
Abstract
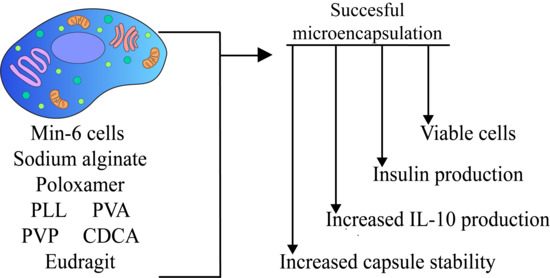
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Encapsulation
2.3. Microcapsule Imaging
2.4. Cell Viability and Insulin Production Analyses
2.5. Inflammatory Profile Analyses
2.6. Cell Biological Activity Analyses
2.7. Chemical and Thermal Analyse
2.8. Statistical Analysis and Graphing
3. Results and Discussion
3.1. Capsule Topographical Analysis and Morphology
3.2. Cell Viability and Insulin Production
3.3. Inflammatory Profile
3.4. Cellular Bioenergetics
3.5. Chemical and Thermal Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. New Biotechnological Microencapsulating Methodology Utilizing Individualized Gradient-Screened Jet Laminar Flow Techniques for Pancreatic β-Cell Delivery: Bile Acids Support Cell Energy-Generating Mechanisms. Mol. Pharm. 2017, 14, 2711–2718. [Google Scholar] [CrossRef]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1508–1519. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic β-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Primary Bile Acid Chenodeoxycholic Acid-Based Microcapsules to Examine β-cell Survival and the Inflammatory Response. BioNanoScience 2016, 6, 103–109. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Influence of biotechnological processes, speed of formulation flow and cellular concurrent stream-integration on insulin production from β-cells as a result of co-encapsulation with a highly lipophilic bile acid. Cell. Mol. Bioeng. 2018, 11, 65–75. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic β-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: Applications in cell-biomaterials, tissue engineering and biotechnological. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Basnet, S.; Ganesan, M.; Pal, T.K. Accelerated stability of sulphamethoxazole microcapsules coated with Eudragit RS 100 and Eudragit RL 100. Boll. Chim. Farm. 2002, 141, 202–209. [Google Scholar]
- Pandey, S.; Vijayendra Swamy, S.M.; Ubaid Ulla, U.M.; Gupta, A.; Patel, H.; Yadav, J.S. Cell Line and Augument Cellular Uptake Study of Statistically Optimized Sustained Release Capecitabine Loaded Eudragit S100/PLGA(poly(lacticco- glycolic acid)) Nanoparticles for Colon Targeting. Curr. Drug Deliv. 2017, 14, 887–899. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.J. Characterization and release behaviors of porous PCL/Eudragit RS microcapsules containing tulobuterol. Colloids Surf. B Biointerfaces 2010, 76, 404–409. [Google Scholar] [CrossRef]
- Azarmi, S.; Farid, J.; Nokhodchi, A.; Bahari-Saravi, S.M.; Valizadeh, H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices. Int. J. Pharm. 2002, 246, 171–177. [Google Scholar] [CrossRef]
- Broughton, R.L.; Sefton, M.V. Effect of capsule permeability on growth of CHO cells in Eudragit RL microcapsules: Use of FITC-dextran as a marker of capsule quality. Biomaterials 1989, 10, 462–465. [Google Scholar] [CrossRef]
- Douglas, J.A.; Sefton, M.V. The permeability of Eudragit RL and Hema-MMA microcapsules to glucose and inulin. Biotechnol. Bioeng. 1990, 36, 653–664. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The influence of stabilized deconjugated ursodeoxycholic acid on polymer-hydrogel system of transplantable NIT-1 cells. Pharm. Res. 2016, 33, 1182–1190. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic beta-cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 194–200. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2016, 6, 17–23. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Alginate-deoxycholic Acid Interaction and Its Impact on Pancreatic Β-Cells and Insulin Secretion and Potential Treatment of Type 1 Diabetes. J. Pharm. Innov. 2016, 11, 156–161. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Biological assessments of encapsulated pancreatic β-cells: Their potential transplantation in diabetes. Cell. Mol. Bioeng. 2016, 9, 530–537. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Darley-Usmar, V.M.; Wu, M.; Jensen, P.B.; Rogers, G.W.; Ferrick, D.A. Bioenergetic Profile Experiment using C2C12 Myoblast Cells. J. Vis. Exp. 2010, 6, e2511. [Google Scholar] [CrossRef]
- Gerencser, A.A.; Neilson, A.; Choi, S.W.; Edman, U.; Yadava, N.; Oh, R.J.; Ferrick, D.A.; Nicholls, D.G.; Brand, M.D. Quantitative Microplate-Based Respirometry with Correction for Oxygen Diffusion. Anal. Chem. 2009, 81, 6868–6878. [Google Scholar] [CrossRef] [Green Version]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. A comprehensive study of novel microcapsules incorporating gliclazide and a permeation enhancing bile acid: Hypoglycemic effect in an animal model of Type-1 diabetes. Drug Deliv. 2016, 23, 2869–2880. [Google Scholar] [CrossRef]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Jones, M.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations. Pharmaceutics 2020, 12, 708. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, T.; Hu, H.; Yuan, S.; Yang, Y.; Wang, R.; Lyu, C.; Yang, F.; Lyu, X. Preparation and Characterization of Microencapsulated Ethylenediamine with Epoxy Resin for Self-healing Composites. Sci. Rep. 2019, 9, 18834. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; Vavassori, P.; Brancaleone, V.; Fiorucci, S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The impact of allylamine-bile acid combinations on cell delivery microcapsules in diabetes. J. Microencapsul. 2016, 33, 569–574. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The effects of Ionic Gelation- Vibrational Jet Flow technique in fabrication of microcapsules incorporating ß-cell: Applications in Type-1 Diabetes. Curr. Diabetes Rev. 2017, 13, 91–96. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Flow vibration-doubled concentric system coupled with low ratio amine to produce bile acid-macrocapsules of β-cells. Ther. Deliv. 2016, 7, 171–178. [Google Scholar] [CrossRef]
- Beyar, R. Challenges in Organ Transplantation. Rambam Maimonides Med. J. 2011, 2, e0049. [Google Scholar] [CrossRef]
- Leitão, C.B.; Cure, P.; Tharavanij, T.; Baidal, D.A.; Alejandro, R. Current challenges in islet transplantation. Curr. Diabetes Rep. 2008, 8, 324–331. [Google Scholar] [CrossRef]
- Mooranian, A.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. The effect of molecular weights of microencapsulating polymers on viability of mouse-cloned pancreatic β-cells: Biomaterials, osmotic forces and potential applications in diabetes treatment. Pharm. Dev. Technol. 2018, 23, 145–150. [Google Scholar] [CrossRef]
- Cieślak, M.; Wojtczak, A.; Cieślak, M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim. Pol. 2015, 62, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Jastroch, M.; Affourtit, C.; Kabra, U.D. Mitochondrial coupling efficiency predicts insulin secretion and classifies dysfunctional properties in pancreatic beta cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, e95. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Sereda, S.B.; Stiles, L.; Elorza, A.; Allister, E.M.; Neilson, A.; Ferrick, D.A.; Wheeler, M.B.; Shirihai, O.S. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS ONE 2012, 7, e33023. [Google Scholar] [CrossRef] [Green Version]
- Dranka, B.P.; Hill, B.G.; Darley-Usmar, V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010, 48, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [Green Version]
- Lazard, D.; Vardi, P.; Bloch, K. Induction of beta-cell resistance to hypoxia and technologies for oxygen delivery to transplanted pancreatic islets. Diabetes/Metab. Res. Rev. 2012, 28, 475–484. [Google Scholar] [CrossRef]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar]
- Rousset, S.; Alves-Guerra, M.C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.M.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. Diabetes 2004, 53 (Suppl. 1), S130–S135. [Google Scholar] [CrossRef] [Green Version]
- Dalgaard, L.T.; Pedersen, O. Uncoupling proteins: Functional characteristics and role in the pathogenesis of obesity and Type II diabetes. Diabetologia 2001, 44, 946–965. [Google Scholar] [CrossRef] [Green Version]
- Malmgren, S.; Nicholls, D.G.; Taneera, J.; Bacos, K.; Koeck, T.; Tamaddon, A.; Wibom, R.; Groop, L.; Ling, C.; Mulder, H.; et al. Tight coupling between glucose and mitochondrial metabolism in clonal β-cells is required for robust insulin secretion. J. Biol. Chem. 2009, 284, 32395–32404. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Neilson, A.; Swift, A.L.; Moran, R.; Tamagnine, J.; Parslow, D.; Armistead, S.; Lemire, K.; Orrell, J.; Teich, J.; et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 2007, 292, C125–C136. [Google Scholar] [CrossRef] [Green Version]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-Nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 7715. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. JBT 2010, 21, 167–193. [Google Scholar]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Elnashar, M.; Arfuso, F.; Al-Salami, H. An in vivo pharmacological study: Variation in tissue-accumulation for the drug probucol as the result of targeted microtechnology and matrix-acrylic acid optimization and stabilization techniques. PLoS ONE 2019, 14, e0214984. [Google Scholar] [CrossRef] [Green Version]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. Morphological, Stability, and Hypoglycemic Effects of New Gliclazide-Bile Acid Microcapsules for Type 1 Diabetes Treatment: The Microencapsulation of Anti-diabetics Using a Microcapsule-Stabilizing Bile Acid. Aaps Pharmscitech 2018, 19, 3009–3018. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.S.; Fang, Z.; Mukkur, T.K.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; et al. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des. Dev. Ther. 2014, 8, 1221. [Google Scholar]





| Primary Chemical Atomic-Bond of % Transmittance (%T) over Wavenumber of Formulations’ Excipients | Primary Chemical Atomic-Bond of % Transmittance (%T) over Wavenumber of Micro/Nano Capsules | |||
|---|---|---|---|---|
| Peak 1 | Peak 2 | |||
| SA | 1043 ± 20 | F1 | 1342 ± 10 | 956 ± 5 |
| CDCA | 17,012 ± 200 | F2 | 1406 ± 15 | 956 ± 20 |
| NM30D® | 333 ± 10 | F3 | 1640 ± 20 | 1022 ± 20 |
| Poloxamer | 1099 ± 20 | F4 | 1342 ± 50 | 1101 ± 40 |
| PVP | 1096 ± 10 | F5 | 1089 ± 40 | 1027 ± 40 |
| PLL | 1405 ± 30 | F6 | 1075 ± 50 | 1701 ± 40 |
| F7 | 1726 ± 50 | 1156 ± 30 | ||
| F8 | 1727 ± 40 | 1167 ± 35 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooranian, A.; Ionescu, C.M.; Walker, D.; Jones, M.; Wagle, S.R.; Kovacevic, B.; Chester, J.; Foster, T.; Johnston, E.; Kuthubutheen, J.; et al. Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems. Biomolecules 2022, 12, 73. https://doi.org/10.3390/biom12010073
Mooranian A, Ionescu CM, Walker D, Jones M, Wagle SR, Kovacevic B, Chester J, Foster T, Johnston E, Kuthubutheen J, et al. Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems. Biomolecules. 2022; 12(1):73. https://doi.org/10.3390/biom12010073
Chicago/Turabian StyleMooranian, Armin, Corina Mihaela Ionescu, Daniel Walker, Melissa Jones, Susbin Raj Wagle, Bozica Kovacevic, Jacqueline Chester, Thomas Foster, Edan Johnston, Jafri Kuthubutheen, and et al. 2022. "Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems" Biomolecules 12, no. 1: 73. https://doi.org/10.3390/biom12010073
APA StyleMooranian, A., Ionescu, C. M., Walker, D., Jones, M., Wagle, S. R., Kovacevic, B., Chester, J., Foster, T., Johnston, E., Kuthubutheen, J., Brown, D., Atlas, M. D., Mikov, M., & Al-Salami, H. (2022). Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems. Biomolecules, 12(1), 73. https://doi.org/10.3390/biom12010073