Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline and Demographic Characteristics
3.2. Serum Acylcarnitines Concentrations
3.3. Acylcarnitine Levels in ACS vs. CCS Patients
3.4. Acylcarnitine Levels in CAD Subgroups
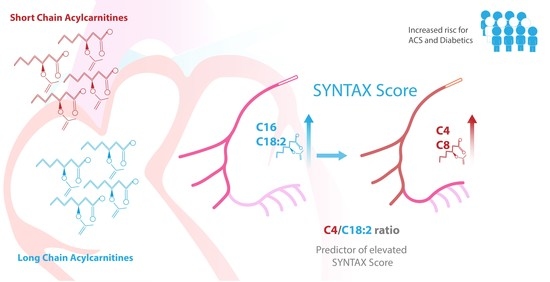
3.5. Correlation of SYNTAX Score with Acylcarnitine Levels in Patients with ACS
3.6. Acylcarnitne Levels in Chronic Kidney Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, L.D.; Gerszten, R.E. Toward New Biomarkers of Cardiometabolic Diseases. Cell Metab. 2013, 18, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Metabolomics for Biomarker Discovery: Moving to the Clinic. BioMed Res. Int. 2015, 2015, 354671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Krause, J.; Zeller, T.; Jones, P.P.; Lamberts, R.R. Long-Chain Acylcarnitines and Cardiac Excitation-Contraction Coupling: Links to Arrhythmias. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma Acylcarnitines and Risk of Cardiovascular Disease: Effect of Mediterranean Diet Interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A Plasma Long-Chain Acylcarnitine Predicts Cardiovascular Mortality in Incident Dialysis Patients. J. Am. Heart Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Alhomida, A.S.; Madani, H.A.; Sobki, S.H. Carnitine and Acylcarnitine Profiles in Dried Blood Spots of Patients with Acute Myocardial Infarction. Metabolomics 2013, 9, 828–838. [Google Scholar] [CrossRef]
- Liepinsh, E.; Makrecka-Kuka, M.; Volska, K.; Kuka, J.; Makarova, E.; Antone, U.; Sevostjanovs, E.; Vilskersts, R.; Strods, A.; Tars, K.; et al. Long-Chain Acylcarnitines Determine Ischaemia/Reperfusion-Induced Damage in Heart Mitochondria. Biochem. J. 2016, 473, 1191–1202. [Google Scholar] [CrossRef]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic Profiling for the Identification of Novel Biomarkers and Mechanisms Related to Common Cardiovascular Diseases: Form and Function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Strand, E.; Pedersen, E.R.; Svingen, G.F.; Olsen, T.; Bjørndal, B.; Karlsson, T.; Dierkes, J.; Njølstad, P.R.; Mellgren, G.; Tell, G.S.; et al. Serum Acylcarnitines and Risk of Cardiovascular Death and Acute Myocardial Infarction in Patients With Stable Angina Pectoris. J. Am. Heart Assoc. 2017, 6, e003620. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Feng, X.-F.; Huang, T.; Luo, H.-H.; Chen, J.-X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.-Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Hruby, A.; Clish, C.B.; Liang, L.; Martínez-González, M.A.; Hu, F.B. Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J. Am. Heart Assoc. 2017, 6, e005705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, M.; Labarthe, F.; Fortier, A.; Bouchard, B.; Legault, J.T.; Bolduc, V.; Rigal, O.; Chen, J.; Ducharme, A.; Crawford, P.A.; et al. Circulating Acylcarnitine Profile in Human Heart Failure: A Surrogate of Fatty Acid Metabolic Dysregulation in Mitochondria and Beyond. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H768–H781. [Google Scholar] [CrossRef] [Green Version]
- Bene, J.; Márton, M.; Mohás, M.; Bagosi, Z.; Bujtor, Z.; Oroszlán, T.; Gasztonyi, B.; Wittmann, I.; Melegh, B. Similarities in Serum Acylcarnitine Patterns in Type 1 and Type 2 Diabetes Mellitus and in Metabolic Syndrome. Ann. Nutr. Metab. 2013, 62, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Kolwicz, S.C.; Purohit, S.; Tian, R. Cardiac Metabolism and Its Interactions With Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; Des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. Assessing Cardiac Metabolism. Circ. Res. 2016, 118, 1659–1701. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of Mitochondrial Long-Chain Fatty Acid Oxidation and the Carnitine Shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Sofidis, G.; Papazoglou, A.S.; Deda, O.; Panteris, E.; Moysidis, D.V.; Stalikas, N.; Kartas, A.; Papadopoulos, A.; Stefanopoulos, L.; et al. Correlation of the Severity of Coronary Artery Disease with Patients’ Metabolic Profile- Rationale, Design and Baseline Patient Characteristics of the CorLipid Trial. BMC Cardiovasc. Disord. 2021, 21, 79. [Google Scholar] [CrossRef]
- Begou, O.A.; Deda, O.; Karagiannidis, E.; Sianos, G.; Theodoridis, G.; Gika, H.G. Development and Validation of a RPLC-MS/MS Method for the Quantification of Ceramides in Human Serum. J. Chromatogr. B 2021, 1175, 122734. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Papazoglou, A.S.; Stalikas, N.; Deda, O.; Panteris, E.; Begou, O.; Sofidis, G.; Moysidis, D.V.; Kartas, A.; Chatzinikolaou, E.; et al. Serum Ceramides as Prognostic Biomarkers of Large Thrombus Burden in Patients with STEMI: A Micro-Computed Tomography Study. J. Pers. Med. 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.-A.; Kappetein, A.P.; Morice, M.-C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An Angiographic Tool Grading the Complexity of Coronary Artery Disease. EuroIntervention 2005, 1, 219–227. [Google Scholar]
- Safarian, H.; Alidoosti, M.; Shafiee, A.; Salarifar, M.; Poorhosseini, H.; Nematipour, E. The SYNTAX Score Can Predict Major Adverse Cardiac Events Following Percutaneous Coronary Intervention. Heart Views Off. J. Gulf Heart Assoc. 2014, 15, 99. [Google Scholar] [CrossRef]
- Khan, M.Y.; Pandit, S.; Guha, S.; Jadhav, U.; Rao, M.S.; Gaurav, K.; Mane, A.; Dubey, A.; Bhagwatkar, H.; Venkataswamy, K.; et al. Demographic Profile, Clinical Characteristics and Medical Management Patterns of Indian Coronary Artery Disease Patients: A Nationwide Urban-Based, Real-World, Retrospective, Observational Electronic Medical Record Study—Report of Baseline Data. Expert Rev. Cardiovasc. Ther. 2021, 19, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Choi, Y.; Back, J.H.; Lee, S.; Jee, S.H.; Park, Y.H. High-Resolution Metabolomics Study Revealing l-Homocysteine Sulfinic Acid, Cysteic Acid, and Carnitine as Novel Biomarkers for High Acute Myocardial Infarction Risk. Metabolism 2020, 104, 154051. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a Peripheral Blood Metabolic Profile with Coronary Artery Disease and Risk of Subsequent Cardiovascular Events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.S.H. Diabetes Mellitus and Coronary Artery Disease. J. Cardiovasc. Risk 1997, 4, 83–90. [Google Scholar] [CrossRef]
- Gunther, S.H.; Khoo, C.M.; Tai, E.-S.; Sim, X.; Kovalik, J.-P.; Ching, J.; Lee, J.J.; van Dam, R.M. Serum Acylcarnitines and Amino Acids and Risk of Type 2 Diabetes in a Multiethnic Asian Population. BMJ Open Diabetes Res. Care 2020, 8, e001315. [Google Scholar] [CrossRef]
- Batchuluun, B.; Rijjal, D.A.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated With Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R.B. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef]
- Blair, H.C.; Sepulveda, J.; Papachristou, D.J. Nature and Nurture in Atherosclerosis: The Roles of Acylcarnitine and Cell Membrane-Fatty Acid Intermediates. Vasc. Pharmacol. 2016, 78, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Clish, C.; Scott, J.; Hanna, D.; Haberlen, S.; Shah, S.; Hodis, H.; Landy, A.; Post, W.; Anastos, K.; et al. Abstract P201: Associations of Plasma Acylcarnitines With Incident Carotid Artery Plaque in Individuals With or at Risk of HIV Infection. Circulation 2018, 137, AP201. [Google Scholar] [CrossRef]
- Rizza, S.; Copetti, M.; Rossi, C.; Cianfarani, M.A.; Zucchelli, M.; Luzi, A.; Pecchioli, C.; Porzio, O.; Di Cola, G.; Urbani, A.; et al. Metabolomics Signature Improves the Prediction of Cardiovascular Events in Elderly Subjects. Atherosclerosis 2014, 232, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Sun, J.-L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline Metabolomic Profiles Predict Cardiovascular Events in Patients at Risk for Coronary Artery Disease. Am. Heart J. 2012, 163, 844–850.e1. [Google Scholar] [CrossRef] [PubMed]
- Naito, R.; Kasai, T. Coronary Artery Disease in Type 2 Diabetes Mellitus: Recent Treatment Strategies and Future Perspectives. World J. Cardiol. 2015, 7, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Mao, Y.; Guo, J.; Zhang, Z.; Li, Y.; Ye, Z.; Ding, Y.; Wang, J.; Jin, M.; Chen, K. The Value of Apolipoprotein B/Apolipoprotein A1 Ratio for Metabolic Syndrome Diagnosis in a Chinese Population: A Cross-Sectional Study. Lipids Health Dis. 2014, 13, 81. [Google Scholar] [CrossRef] [Green Version]


| Sex | CAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Female | Male | CCS | ACS | |||||||
| N | N% | N | N% | # p | N | N% | N | N% | # p | ||
| Hypertension | No | 84 | 21.10 | 314 | 78.90 | 0.0001 | 149 | 37.40 | 249 | 62.60 | 0.0001 |
| Yes | 171 | 30.50 | 389 | 69.50 | 276 | 49.30 | 284 | 50.70 | |||
| Diabetes Mellitus | No | 161 | 25.10 | 481 | 74.90 | 0.124 | 285 | 44.40 | 357 | 55.60 | 0.979 |
| Yes | 94 | 29.70 | 222 | 70.30 | 140 | 44.30 | 176 | 55.70 | |||
| Dyslipidemia | No | 145 | 24.40 | 449 | 75.60 | 0.046 | 232 | 39.10 | 362 | 60.90 | 0.0001 |
| Yes | 110 | 30.30 | 253 | 69.70 | 193 | 53.20 | 170 | 46.80 | |||
| Smoking | No | 190 | 35.50 | 345 | 64.50 | 0.0001 | 286 | 53.50 | 249 | 46.50 | 0.0001 |
| Yes | 65 | 15.40 | 358 | 84.60 | 139 | 32.90 | 284 | 67.10 | |||
| Age groups | 65< | 99 | 19.60 | 405 | 80.40 | 0.0001 | 201 | 39.90 | 303 | 60.10 | 0.003 |
| 65> | 155 | 34.30 | 297 | 65.70 | 224 | 49.60 | 228 | 50.40 | |||
| Chronic Kidney Disease | No | 196 | 24.00 | 622 | 76.00 | 0.0001 | 374 | 45.70 | 444 | 54.30 | 0.005 |
| Yes | 55 | 43.30 | 72 | 56.70 | 41 | 32.30 | 86 | 67.70 | |||
| SYNTAX Score Groups | 0 | 103 | 37.20 | 174 | 62.80 | 0.001 | 192 | 69.30 | 85 | 30.70 | 0.0001 |
| 1 to 22 | 99 | 21.00 | 372 | 79.00 | 167 | 35.50 | 304 | 64.50 | |||
| >22 | 53 | 25.20 | 157 | 74.80 | 66 | 31.40 | 144 | 68.60 | |||
| CAD Groups | |||||||||||
| Baseline Characteristics | NSTEMI(α) | STEMI(β) | UA (γ) | SA(δ) | |||||||
| N | N% | N | N% | N | N% | N | N% | * p (pair) | |||
| Hypertension | No | 63 | 15.80 | 129 | 32.40 | 57 | 14.30 | 149 | 37.40 | 0.005 (β–α), <0.001 (β–γ), <0.001 (β–δ), | |
| Yes | 107 | 19.10 | 93 | 16.60 | 84 | 15.00 | 276 | 49.30 | |||
| Diabetes Mellitus | No | 111 | 17.30 | 160 | 24.90 | 86 | 13.40 | 285 | 44.40 | 0.164 | |
| Yes | 59 | 18.70 | 62 | 19.60 | 55 | 17.40 | 140 | 44.30 | |||
| Dyslipidemia | No | 104 | 17.50 | 166 | 27.90 | 92 | 15.50 | 232 | 39.10 | 0.045 (β–α), >0.001 (β–δ), | |
| Yes | 65 | 17.90 | 56 | 15.40 | 49 | 13.50 | 193 | 53.20 | |||
| Smoking | No | 78 | 14.60 | 94 | 17.60 | 77 | 14.40 | 286 | 53.50 | >0.001(δ–α), >0.001(δ–β) | |
| Yes | 92 | 21.70 | 128 | 30.30 | 64 | 15.10 | 139 | 32.90 | |||
| Age groups | 65< | 93 | 18.50 | 143 | 28.40 | 67 | 13.30 | 201 | 39.90 | 0.013 (β–γ), >0.001 (β–δ), | |
| 65> | 76 | 16.80 | 79 | 17.50 | 73 | 16.20 | 224 | 49.60 | |||
| Chronic Kidney Disease | No | 132 | 16.10 | 191 | 23.30 | 121 | 14.80 | 374 | 45.70 | <0.001 (δ–α) | |
| Yes | 38 | 29.90 | 29 | 22.80 | 19 | 15.00 | 41 | 32.30 | |||
| SYNTAX Score Groups | 0 | 25 | 9.00 | 11 | 4.00 | 49 | 17.70 | 192 | 69.30 | <0.001 (δ–α), <0.001 (δ–β), <0.001 (γ–α), <0.001 (γ–β) | |
| 1 to 22 | 90 | 19.10 | 151 | 32.10 | 63 | 13.40 | 167 | 35.50 | |||
| >22 | 55 | 26.20 | 60 | 28.60 | 29 | 13.80 | 66 | 31.40 | |||
| Sex | CAD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | CCS | ACS | |||||||||||
| Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | # p-Value | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | #p | |
| BMI | 28.1 | 27.5 | 28.8 | 27.9 | 27.7 | 28.4 | 0.407 | 28.27 | 27.8 | 28.7 | 27.8 | 27.5 | 28.4 | 0.127 |
| CHOL | 163 | 155 | 171 | 158 | 154 | 163 | 0.073 | 160 | 156 | 166 | 158 | 154 | 165 | 0.96 |
| TG | 127 | 119 | 133 | 124 | 120 | 130 | 0.891 | 122 | 116 | 128 | 129 | 122 | 135 | 0.039 |
| HDL | 45 | 42 | 46 | 39 | 39 | 41 | 0 | 43 | 42 | 46 | 39 | 39 | 41 | 0 |
| LDL | 87 | 81 | 94 | 89 | 86 | 93 | 0.91 | 87 | 83 | 92 | 89 | 85 | 94 | 0.104 |
| TnThs | 27 | 20 | 41 | 41 | 30 | 52 | 0.003 | 14 | 13 | 16 | 250 | 180 | 357 | 0 |
| LVEF (%) | 0.55 | 0.55 | 0.60 | 0.55 | 0.55 | 0.60 | 0.121 | 0.60 | 0.60 | 0.65 | 0.50 | 0.50 | 0.55 | 0 |
| GFR | 83.6 | 78.7 | 88 | 96.6 | 94 | 99.3 | 0 | 89.2 | 86.3 | 93.6 | 95.8 | 92.4 | 98.7 | 0.389 |
| CAD Groups | ||||||||||||||
| NSTEMI (α) | STEMI (β) | UA (γ) | SA (δ) | |||||||||||
| Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | * p (pair) | ||
| BMI | 27.7 | 27 | 28.4 | 28.1 | 27.7 | 28.7 | 27.7 | 26.7 | 29 | 28.27 | 27.8 | 28.7 | 0.189 | |
| CHOL | 155 | 148 | 169 | 162 | 154 | 169 | 155 | 151 | 167 | 160 | 156 | 166 | 0.648 | |
| TG | 130 | 120 | 142 | 125 | 116 | 136 | 134 | 117 | 142 | 122 | 116 | 128 | 0.159 | |
| HDL | 38 | 37 | 40 | 38 | 36 | 40 | 40 | 38 | 43 | 43 | 42 | 46 | >0.001 (β–δ), >0.001 (α–δ) | |
| LDL | 85 | 78 | 101 | 94 | 90 | 106 | 86 | 81 | 92 | 87 | 83 | 92 | 0.024 (γ–β) | |
| TnThs | 244 | 175 | 347 | 1409 | 1175 | 1915 | 18 | 16 | 26 | 14 | 13 | 16 | >0.001 (δ–α), >0.001 (δ–β), >0.001 (δ–γ,) >0.001 (γ–α), >0.001 (γ–β), >0.001 (α–β) | |
| LVEF (%) | 0.50 | 0.50 | 0.55 | 0.45 | 0.45 | 0.50 | 0.55 | 0.55 | 0.60 | 0.60 | 0.60 | 0.65 | >0.001 (δ–α), >0.001 (δ–β), 0.015 (γ–α), >0.001 (γ–β), >0.001 (α–β), | |
| GFR | 91.8 | 84.4 | 98.3 | 98.1 | 92.8 | 101.4 | 96.1 | 88.5 | 100 | 89.2 | 86.3 | 93.6 | 0.086 | |
| CAD | |||||||
|---|---|---|---|---|---|---|---|
| CCS | ACS | ||||||
| Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | * p | |
| C8 | 63.06 | 58.68 | 68.55 | 54.75 | 51.21 | 57.68 | 0.012 |
| C10 | 106.12 | 96.74 | 116.37 | 88.51 | 83.51 | 93.92 | 0.007 |
| C16 | 63.21 | 60.85 | 65.63 | 59.97 | 57.66 | 61.97 | 0.018 |
| C18:1 | 92.54 | 88.15 | 97.76 | 84.43 | 80.05 | 89.91 | 0.011 |
| C18:2 | 60.22 | 57.89 | 63.29 | 51.89 | 50.44 | 54.67 | <0.001 |
| Non-DM Patients | DM Patients (HBA1C > 6.5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | ↓95.0% CIs | ↑95.0% CIs | * p | Median | ↓95.0% CIs | ↑95.0% CIs | * p | ||
| C2 | CCS | 2957.11 | 2734.56 | 3089.26 | 0.321 | 2761.05 | 2529.49 | 2964.76 | 0.088 |
| ACS | 3036.99 | 2817.51 | 3365.70 | 3289.10 | 2976.97 | 3801.96 | |||
| C3 | CCS | 172.44 | 162.80 | 184.61 | 0.979 | 166.81 | 155.35 | 177.16 | 0.310 |
| ACS | 185.16 | 172.28 | 203.39 | 191.23 | 177.00 | 214.25 | |||
| C4 | CCS | 37.76 | 34.94 | 40.46 | 0.597 | 37.21 | 35.39 | 40.48 | 0.876 |
| ACS | 42.47 | 37.42 | 48.06 | 40.62 | 37.34 | 45.16 | |||
| C5 | CCS | 25.91 | 24.91 | 28.07 | 0.463 | 26.17 | 24.95 | 28.33 | 0.351 |
| ACS | 25.25 | 24.02 | 29.21 | 26.81 | 24.57 | 30.87 | |||
| C6 | CCS | 29.84 | 28.47 | 31.34 | 0.078 | 27.32 | 25.44 | 29.10 | 0.522 |
| ACS | 30.35 | 28.07 | 33.39 | 30.47 | 28.46 | 33.44 | |||
| C8 | CCS | 61.50 | 56.90 | 67.91 | 0.008 | 52.59 | 49.35 | 55.94 | 0.573 |
| ACS | 67.82 | 56.94 | 77.31 | 59.00 | 53.41 | 65.99 | |||
| C10 | CCS | 103.06 | 95.47 | 112.23 | 0.004 | 84.57 | 79.88 | 91.77 | 0.612 |
| ACS | 117.07 | 92.22 | 132.23 | 97.62 | 86.47 | 115.32 | |||
| C12 | CCS | 29.95 | 27.55 | 31.44 | 0.026 | 26.48 | 24.49 | 28.74 | 0.995 |
| ACS | 29.88 | 27.29 | 33.30 | 29.71 | 27.15 | 32.21 | |||
| C14 | CCS | 19.26 | 18.12 | 19.86 | 0.011 | 17.54 | 16.81 | 18.74 | 0.715 |
| ACS | 19.05 | 17.91 | 20.53 | 18.90 | 17.62 | 20.53 | |||
| C16 | CCS | 64.79 | 61.80 | 66.64 | 0.012 | 60.15 | 57.23 | 62.77 | 0.578 |
| ACS | 61.00 | 58.42 | 65.28 | 59.54 | 56.19 | 63.82 | |||
| C18 | CCS | 19.22 | 18.19 | 19.91 | 0.038 | 18.11 | 17.49 | 18.77 | 0.758 |
| ACS | 18.74 | 17.75 | 19.77 | 18.76 | 17.63 | 19.32 | |||
| C18:1 | CCS | 93.41 | 87.84 | 99.43 | 0.003 | 82.36 | 77.78 | 88.15 | 0.813 |
| ACS | 92.53 | 86.95 | 100.00 | 89.91 | 81.61 | 97.51 | |||
| C18:2 | CCS | 59.96 | 56.67 | 63.31 | <0.001 | 50.89 | 49.07 | 53.70 | 0.202 |
| ACS | 61.35 | 57.75 | 67.58 | 54.99 | 51.18 | 60.46 | |||
| CAD Groups | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NSTEMI (α) | STEMI (β) | UA (γ) | SA (δ) | ||||||||||
| Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | * p (Pair) | |
| C5 | 24.79 | 23.46 | 28.80 | 29.08 | 26.36 | 30.73 | 25.13 | 22.86 | 27.70 | 25.72 | 24.95 | 27.50 | 0.026 (δ–γ) |
| C10 | 89.88 | 78.25 | 105.42 | 86.53 | 79.36 | 94.45 | 91.50 | 83.49 | 110.42 | 106.09 | 96.74 | 116.37 | 0.019 (δ–β) |
| C16 | 58.38 | 55.03 | 63.64 | 58.29 | 55.82 | 60.89 | 62.90 | 60.82 | 66.52 | 63.21 | 60.85 | 65.63 | 0.012 (δ–β) |
| C18:1 | 85.82 | 78.64 | 94.91 | 82.80 | 76.43 | 88.61 | 91.30 | 79.31 | 97.06 | 92.53 | 88.15 | 97.76 | 0.013 (δ–β) |
| C18:2 | 54.86 | 50.74 | 59.54 | 50.26 | 47.34 | 52.41 | 53.75 | 50.00 | 60.48 | 60.21 | 57.89 | 63.29 | >0.001 (δ–β) |
| SYNTAX Score Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (a) | 1 to 22 (b) | >22 (c) | ||||||||
| Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | Median | ↓95.0% CIs | ↑95.0% CIs | * p (Pair) | |
| C4 | 36.96 | 34.21 | 40.46 | 37.95 | 35.6 | 40.18 | 45.16 | 38.94 | 49.61 | 0.002 (a–c) 0.005 (b–c) |
| C5 | 25.25 | 23.99 | 26.36 | 26.41 | 24.95 | 28.63 | 27.82 | 25.34 | 30.79 | 0.024 (a–c) |
| C16 | 65.18 | 62.57 | 67.9 | 60.28 | 57.95 | 62.48 | 59.27 | 56.28 | 61.94 | 0.031 (c–a) 0.044 (b–a) |
| C18:2 | 60.48 | 56.37 | 64.61 | 53.83 | 51.35 | 56.62 | 53.28 | 49.37 | 57.57 | 0.019 (c–a) 0.012 (b–a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deda, O.; Panteris, E.; Meikopoulos, T.; Begou, O.; Mouskeftara, T.; Karagiannidis, E.; Papazoglou, A.S.; Sianos, G.; Theodoridis, G.; Gika, H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules 2022, 12, 354. https://doi.org/10.3390/biom12030354
Deda O, Panteris E, Meikopoulos T, Begou O, Mouskeftara T, Karagiannidis E, Papazoglou AS, Sianos G, Theodoridis G, Gika H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules. 2022; 12(3):354. https://doi.org/10.3390/biom12030354
Chicago/Turabian StyleDeda, Olga, Eleftherios Panteris, Thomas Meikopoulos, Olga Begou, Thomai Mouskeftara, Efstratios Karagiannidis, Andreas S. Papazoglou, Georgios Sianos, Georgios Theodoridis, and Helen Gika. 2022. "Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease" Biomolecules 12, no. 3: 354. https://doi.org/10.3390/biom12030354
APA StyleDeda, O., Panteris, E., Meikopoulos, T., Begou, O., Mouskeftara, T., Karagiannidis, E., Papazoglou, A. S., Sianos, G., Theodoridis, G., & Gika, H. (2022). Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules, 12(3), 354. https://doi.org/10.3390/biom12030354