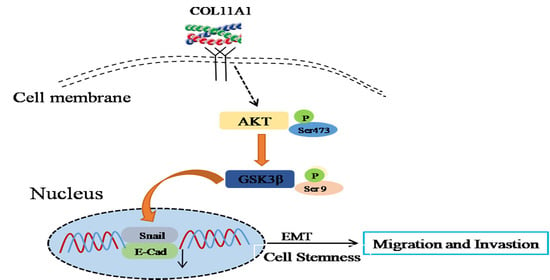
COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Transfection, and Treatment
2.2. Transwell Assay
2.3. Western Blotting
2.4. Chromatin Immunoprecipitation (ChIP)
2.5. Immunofluorescence
2.6. Wound-Healing Assays
2.7. Real-Time PCR
2.8. Flow Cytometry Analysis and Sorting
2.9. Adherent Assay
2.10. Colony Formation Assay
2.11. Statistical Analysis
3. Results
3.1. Enhanced Migration and Invasion Abilities Induced by COL11A1 in Pancreatic Cancer Cells
3.2. COL11A1 Modulated the EMT-likePhenotypic Changes and Matrix Metalloproteinase (MMP)-2/9Expression Levels
3.3. COL11A1 Induced the Migration and Invasion of Pancreatic Cancer Cells via the Activation of theAKT/GSK-3β/Snail Pathway
3.4. AKT/GSK-3β/Snail Axis Was Pivotal for COL11A1-Induced EMT
3.5. COL11A1 Modulated the Cell Stemness Efficiency by Regulating the AKT/GSK-3β/Snail Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Elkholy, S.; El-Sayed, R.; Wifi, M.N.; El-Nady, M.; El-Nabawi, W.; El-Dayem, W.A.; Radwan, M.I.; Farag, A.; El-Sherif, Y.; et al. Real time endoscopic ultrasound elastography and strain ratio in the diagnosis of solid pancreatic lesions. World J. Gastroenterol. 2017, 23, 5962–5968. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Lauer, J.L.; Fields, G.B. Collagen in Cancer. In Cancer Drug Discovery & Development; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Kleinert, R.; Prenzel, K.; Stoecklein, N.; Alakus, H.; Bollschweiler, E.; Holscher, A.; Warnecke-Eberz, U. Gene Expression of Col11A1 Is a Marker Not only for Pancreas Carcinoma But also for Adenocarcinoma of the Papilla of Vater, Discriminating Between Carcinoma and Chronic Pancreatitis. Anticancer Res. 2015, 35, 6153–6158. [Google Scholar] [PubMed]
- Wang, H.; Ren, R.; Yang, Z.; Cai, J.; Du, S.; Shen, X. The COL11A1/Akt/CREB signaling axis enables mitochondrial-mediated apoptotic evasion to promote chemoresistance in pancreatic cancer cells through modulating BAX/BCL-2 function. J. Cancer 2021, 12, 1406–1420. [Google Scholar] [CrossRef]
- Shen, L.; Yang, M.; Lin, Q.; Zhang, Z.; Zhu, B.; Miao, C. COL11A1 is overexpressed in recurrent non-small cell lung cancer and promotes cell proliferation, migration, invasion and drug resistance. Oncol. Rep. 2016, 36, 877–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Li, J.; Lin, J.; Zhuo, W.; Si, J. COL11A1 is overexpressed in gastric cancer tissues and regulates proliferation, migration and invasion of HGC-27 gastric cancer cells in vitro. Oncol. Rep. 2017, 37, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Zavadil, J.; Bottinger, E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef] [Green Version]
- Savagner, P.; Yamada, K.M.; Thiery, J.P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J. Cell Biol. 1997, 137, 1403–1419. [Google Scholar] [CrossRef]
- Qi, X.; Sun, L.; Wan, J.; Xu, R.; He, S.; Zhu, X. Tensin4 promotes invasion and migration of gastric cancer cells via regulating AKT/GSK-3beta/snail signaling pathway. Pathol. Res. Pract. 2020, 216, 153001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, M.; Lin, X.H.; Liu, H.H.; Chen, J.; Gao, D.M.; Cui, J.F.; Ren, Z.G.; Chen, R.X. Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment. BMC Cancer 2018, 18, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 2019, 63, 109377. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Tu, H.; Li, J.; Lin, L.; Wang, L. COL11A1 Was Involved in Cell Proliferation, Apoptosis and Migration in Non-Small Cell Lung Cancer Cells. J. Investig. Surg. 2021, 34, 664–669. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Rada, M.; Nallanthighal, S.; Cha, J.; Ryan, K.; Sage, J.; Eldred, C.; Ullo, M.; Orsulic, S.; Cheon, D.J. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 2018, 37, 4809–4820. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chang, T.H.; Huang, Y.F.; Chen, C.C.; Chou, C.Y. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPbeta pathway and PDK1 stabilization. Oncotarget 2015, 6, 23748–23763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, J.D.; Yan, L.J.; Zhao, X.P. PDGF-D/PDGFRbeta promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem. Biophys. Res. Commun. 2016, 478, 845–851. [Google Scholar] [CrossRef]
- Saegusa, M.; Hashimura, M.; Kuwata, T.; Okayasu, I. Requirement of the Akt/beta-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of slug. Am. J. Pathol. 2009, 174, 2107–2115. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, E.; Oki, E.; Egashira, A.; Sadanaga, N.; Morita, M.; Kakeji, Y.; Maehara, Y. Deregulation of the Akt pathway in human cancer. Curr. Cancer Drug Targets 2008, 8, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Lei, J.Q.; Guo, S.L.; Zhao, D.; Yue, H.M.; Yu, Q. The CNPY2 enhances epithelial-mesenchymal transition via activating the AKT/GSK3beta pathway in non-small cell lung cancer. Cell Biol. Int. 2018, 42, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; Faggion, B.; Balboni, B.; Carbone, D.; Peters, G.J.; Diana, P.; Assaraf, Y.G.; Giovannetti, E. GSK3beta as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist. Updates 2021, 58, 100779. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Chen, X.H.; Zhang, G.; Liu, Z.C.; Wu, N.; Wang, H.; Qi, Y.F.; Wang, H.S.; Cai, S.H.; Du, J. CCL21 Facilitates Chemoresistance and Cancer Stem Cell-Like Properties of Colorectal Cancer Cells through AKT/GSK-3beta/Snail Signals. Oxid. Med. Cell. Longev. 2016, 2016, 5874127. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.C.; Chen, X.H.; Song, H.X.; Wang, H.S.; Zhang, G.; Wang, H.; Chen, D.Y.; Fang, R.; Liu, H.; Cai, S.H.; et al. Snail regulated by PKC/GSK-3beta pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014, 358, 491–502. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Z.; Jin, S.; Xu, K.; Zhang, H.; Xu, J.; Sun, Q.; Wang, J. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3beta/Snail signaling. Cancer Sci. 2018, 109, 1414–1427. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Yokoi, A.; Hashimura, M.; Oguri, Y.; Akiya, M.; Saegusa, M. TGF-beta-mediated LEFTY/Akt/GSK-3beta/Snail axis modulates epithelial-mesenchymal transition and cancer stem cell properties in ovarian clear cell carcinomas. Mol. Carcinog. 2018, 57, 957–967. [Google Scholar] [CrossRef]
- Scheel, C.; Weinberg, R.A. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin. Cancer Biol. 2012, 22, 396–403. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Wang, L.; Huang, Y.; Xu, Y.; Xu, L.; Guo, Y.; Lu, J.; Li, X.; Zhu, M.; et al. MicroRNA-30b targets Snail to impede epithelial-mesenchymal transition in pancreatic cancer stem cells. J. Cancer 2018, 9, 2147–2159. [Google Scholar] [CrossRef] [Green Version]
- Ota, I.; Masui, T.; Kurihara, M.; Yook, J.I.; Mikami, S.; Kimura, T.; Shimada, K.; Konishi, N.; Yane, K.; Yamanaka, T.; et al. Snail-induced EMT promotes cancer stem cell-like properties in head and neck cancer cells. Oncol. Rep. 2016, 35, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Meller, R.; Minami, M.; Cameron, J.A.; Impey, S.; Chen, D.; Lan, J.Q.; Henshall, D.C.; Simon, R.P. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J. Cereb. Blood Flow Metab. 2005, 25, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Ding, J.; Li, O.; He, Q.; Ke, M.; Zhu, M.; Liu, L.; Ou, W.B.; He, Y.; Wu, Y. Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection. Stem Cell Res. Ther. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Lee, Y.; Lee, H.; Kim, J.W.; Hwang, J.H.; Kim, J.; Yoon, Y.S.; Han, H.S.; Kim, H. The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumour Biol. 2017, 39, 1010428317718403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Jin, H.; Zhang, J.; Tan, X. Integrated whole genome microarray analysis and immunohistochemical assay identifies COL11A1, GJB2 and CTRL as predictive biomarkers for pancreatic cancer. Cancer Cell Int. 2018, 18, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, H.; Liang, C.; Li, Z.; An, D.; Ren, S.; Yue, C.; Wu, J. DcR3 induces proliferation, migration, invasion, and EMT in gastric cancer cells via the PI3K/AKT/GSK-3beta/beta-catenin signaling pathway. OncoTargets Ther. 2018, 11, 4177–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, A.; Perez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Guo, L.; Sun, C.; Xu, S.; Xu, Y.; Dong, Q.; Zhang, L.; Li, W.; Wang, X.; Ying, G.; Guo, F. Knockdown of long non-coding RNA linc-ITGB1 inhibits cancer stemness and epithelial-mesenchymal transition by reducing the expression of Snail in non-small cell lung cancer. Thorac. Cancer 2019, 10, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kaur, H.; De, R.; Srinivasan, R.; Pal, A.; Bhattacharyya, S. Knockdown of E-cadherin induces cancer stem-cell-like phenotype and drug resistance in cervical cancer cells. Biochem. Cell Biol. 2021, 99, 587–595. [Google Scholar] [CrossRef]
- Whatcott, C.J.; Diep, C.H.; Jiang, P.; Watanabe, A.; LoBello, J.; Sima, C.; Hostetter, G.; Shepard, H.M.; Von Hoff, D.D.; Han, H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin. Cancer Res. 2015, 21, 3561–3568. [Google Scholar] [CrossRef] [Green Version]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Nerenberg, P.S.; Salsas-Escat, R.; Stultz, C.M. Collagen--a necessary accomplice in the metastatic process. Cancer Genom. Proteom. 2007, 4, 319–328. [Google Scholar]
- Fischer, H.; Stenling, R.; Rubio, C.; Lindblom, A. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis 2001, 22, 875–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, G.V.; Zhao, N.; Den Hollander, P.; Toneff, M.J.; Joseph, R.; Pietila, M.; Taube, J.H.; Sarkar, T.R.; Ramirez-Pena, E.; Werden, S.J.; et al. GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Barbera, M.J.; Puig, I.; Dominguez, D.; Julien-Grille, S.; Guaita-Esteruelas, S.; Peiro, S.; Baulida, J.; Franci, C.; Dedhar, S.; Larue, L.; et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene 2004, 23, 7345–7354. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Chen, Y.; Li, Y.; Lyu, X.; Cui, J.; Cheng, Y.; Zheng, T.; Zhao, L.; Zhao, G. Resveratrol Suppresses Epithelial-Mesenchymal Transition in GBM by Regulating Smad-Dependent Signaling. BioMed Res. Int. 2019, 2019, 1321973. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Lv, R.; Qi, W.; Wu, D.; Xu, Y.; Liu, W.; Mou, Y.; Wang, L. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer. PLoS ONE 2014, 9, e87409. [Google Scholar] [CrossRef]
- Hermann, P.C.; Bhaskar, S.; Cioffi, M.; Heeschen, C. Cancer stem cells in solid tumors. Semin. Cancer Biol. 2010, 20, 77–84. [Google Scholar] [CrossRef]
- Ma, C.; Huang, T.; Ding, Y.C.; Yu, W.; Wang, Q.; Meng, B.; Luo, S.X. MicroRNA-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6533–6539. [Google Scholar]








| Gene | siRNA Sequences (5′ to 3′) |
|---|---|
| siCOL11A1-1 | 5′-CUCCAGUUGAUGUACUAAATT-3′ |
| siCOL11A1-1 | 5′-CCAGAGGAUAUAAUCGAAUTT-3′ |
| siSnail-1 | 5′-GCGAGCUGCAGGACUCUAA-3′ |
| siSnail-2 | 5′-GUGACUAACUACUGCAAUAA-3′ |
| siGSK-3β-1 | 5′-GGGCCUUAUAUACUCUAAA-3′ |
| siGSK-3β-2 | 5′-GCCUCAAAGUAGUCCAUAU-3′ |
| Name | Company | Catalog Number | Antibody Concentration |
|---|---|---|---|
| E-cad | Cell Signaling Technology, Massachusetts USA | 14472S | 1:1000 |
| N-cad | Cell Signaling Technology, USA | 13116S | 1:1000 |
| VIM | Cell Signaling Technology, USA | 5741S | 1:1000 |
| Snail | Cell Signaling Technology, USA | 3879S | 1:1000 |
| AKT | Proteintech, Chicago, USA | 60203-2-Ig | 1:2000 |
| p-AKTSer473 | Cell Signaling Technology, USA | 4060S | 1:2000 |
| MMP-2 | Cell Signaling Technology, USA | 40994S | 1:1000 |
| MMP-9 | Cell Signaling Technology, USA | 13667S | 1:1000 |
| GSK-3β | Cell Signaling Technology, USA | 12456S | 1:1000 |
| p-GSK-3βSer9 | Cell Signaling Technology, USA | 5558S | 1:1000 |
| CD24 | Proteintech, USA | 18330-1-AP | 1:500 |
| CD44 | Proteintech, USA | 60224-1-Ig | 1:1000 |
| β-actin | Sungene Biotech, Tianjin, China | KM9001 | 1:5000 |
| Genes | Primer Sequences |
|---|---|
| CD24 | F: 5′-TGCTCCTACCCACGCAGATT-3′R: 5′-GGCCAACCCAGAGTTGGAA-3′ |
| CD44 | F: 5′-CACAATCCAGGCAACTCCTA-3′R: 5′-TACTCTGCTGCGTTGTCATT-3′ |
| GAPDH | F: 5′-TGCACCACCAACTGCTTAGC-3′R: 5′-GGCATGGACTGTGGTCATGAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhou, H.; Ni, H.; Shen, X. COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway. Biomolecules 2022, 12, 391. https://doi.org/10.3390/biom12030391
Wang H, Zhou H, Ni H, Shen X. COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway. Biomolecules. 2022; 12(3):391. https://doi.org/10.3390/biom12030391
Chicago/Turabian StyleWang, Hui, Huichao Zhou, Hong Ni, and Xiaohong Shen. 2022. "COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway" Biomolecules 12, no. 3: 391. https://doi.org/10.3390/biom12030391
APA StyleWang, H., Zhou, H., Ni, H., & Shen, X. (2022). COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway. Biomolecules, 12(3), 391. https://doi.org/10.3390/biom12030391