Impacts of Bacteriostatic and Bactericidal Antibiotics on the Mitochondria of the Age-Related Macular Degeneration Cybrid Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethic Statement
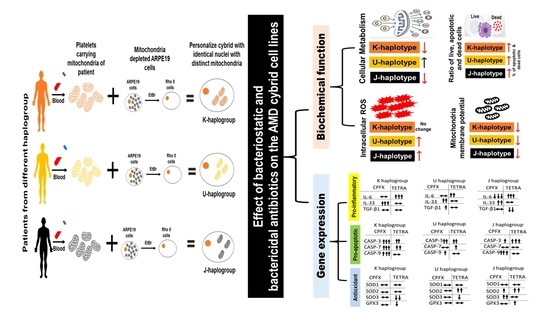
2.2. Cybrids Creation
2.3. Cybrids Culture Conditions
2.4. Intracellular Level of Reactive Oxygen Species (ROS Assay)
2.5. Mitochondria Membrane Potential (ΔψM) (JC-1 Assay)
2.6. Cellular Metabolism Assay (MTT Assay)
2.7. The Ratio of Live, Apoptotic and Dead Cells (Flow Cytometry)
2.8. Isolation of RNA and cDNA Amplification
2.9. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analyses
3. Results
3.1. ROS Levels
3.2. Alterations of Mitochondrial Membrane Potential (ΔψM)
3.3. Changes in Cellular Metabolism (MTT Assay)
3.4. Ratio of Apoptosis and Dead Cells (Flowcytometry)
3.5. Alterations of Pro-Inflammatory, Pro-Apoptotic and Antioxidant Enzymes Genes
3.5.1. K Cybrids
3.5.2. U Cybrids
3.5.3. J Cybrids
4. Discussion
5. Effects of CPFX on AMD Cybrids
Effects of TETRA on AMD Cybrids
6. Conclusions
7. Strength and Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. The resistance tsunami, antimicrobial stewardship, and the golden age of microbiology. Vet. Microbiol. 2014, 171, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Pennefather, P.M.; Kaye, S.B.; Hart, C.A. Fluoroquinolones. Drugs 2001, 61, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-S.; Fisher, L.M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: Selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob. Agents Chemother. 1997, 41, 471–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, T.; Cook, J. Fluoroquinolones and tendinopathy: A guide for athletes and sports clinicians and a systematic review of the literature. J. Athl. Train. 2014, 49, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Rosmarakis, E.S. Arrhythmias associated with fluoroquinolone therapy. Int. J. Antimicrob. Agents 2007, 29, 374–379. [Google Scholar] [CrossRef]
- Samarakoon, N.; Harrisberg, B.; Ell, J. Ciprofloxacin-induced toxic optic neuropathy. Clin. Exp. Ophthalmol. 2007, 35, 102–104. [Google Scholar] [CrossRef]
- Fife, D.; Zhu, V.; Voss, E.; Levy-Clarke, G.; Ryan, P. Exposure to oral fluoroquinolones and the risk of retinal detachment: Retrospective analyses of two large healthcare databases. Drug Saf. 2014, 37, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.C. Tetracyclines: Mode of action and their bacterial mechanisms of resistance. Bact. Resist. Antibiot. Mol. Man 2019, 101–124. [Google Scholar]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.M.; McCulley, J.P.; Silvany, R.E.; Meyer, D.R. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2970–2975. [Google Scholar]
- Sandler, C.; Ekokoski, E.; Lindstedt, K.A.; Vainio, P.; Finel, M.; Sorsa, T.; Kovanen, P.T.; Golub, L.; Eklund, K.K. Chemically modified tetracycline (CMT)-3 inhibits histamine release and cytokine production in mast cells: Possible involvement of protein kinase C. Inflamm. Res. 2005, 54, 304–312. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Zakeri, B.; Wright, G.D. Chemical biology of tetracycline antibiotics. Biochem. Cell Biol. 2008, 86, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Etminan, M. Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacotherapy 2020, 40, 487. [Google Scholar] [CrossRef] [Green Version]
- Sandler, C.; Nurmi, K.; Lindstedt, K.A.; Sorsa, T.; Golub, L.M.; Kovanen, P.T.; Eklund, K.K. Chemically modified tetracyclines induce apoptosis in cultured mast cells. Int. Immunopharmacol. 2005, 5, 1611–1621. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Rogers, R.S., III; Sheridan, P.J. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int. J. Dermatol. 2004, 43, 709–715. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Tetracyclines: Light-activated antibiotics? Future Med. Chem. 2019, 11, 2427–2445. [Google Scholar] [CrossRef]
- Wallace, I.; Krupin, T.; Stone, R.; Moolchandani, J. The ocular hypotensive effects of demeclocycline, tetracycline and other tetracycline derivatives. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1594–1598. [Google Scholar]
- Tielens, A.G.; Rotte, C.; van Hellemond, J.J.; Martin, W. Mitochondria as we don’t know them. Trends Biochem. Sci. 2002, 27, 564–572. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.W.; Burger, G.; Lang, B.F. Mitochondrial evolution. Science 1999, 283, 1476–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, G.; Karlberg, O.; Canbäck, B.; Kurland, C.G. On the origin of mitochondria: A genomics perspective. Philos. Trans. R. Soc. London. Ser. B: Biol. Sci. 2003, 358, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The similarities between human mitochondria and bacteria in the context of structure, genome, and base excision repair system. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 2013, 5, 192ra185. [Google Scholar] [CrossRef] [Green Version]
- Kenney, M.C.; Atilano, S.R.; Boyer, D.; Chwa, M.; Chak, G.; Chinichian, S.; Coskun, P.; Wallace, D.C.; Nesburn, A.B.; Udar, N.S. Characterization of retinal and blood mitochondrial DNA from age-related macular degeneration patients. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4289–4297. [Google Scholar] [CrossRef]
- Salimiaghdam, N.; Singh, L.; Schneider, K.; Nalbandian, A.; Chwa, M.; Atilano, S.R.; Bao, A.; Kenney, M.C. Potential adverse effects of ciprofloxacin and tetracycline on ARPE-19 cell lines. BMJ Open Ophthalmol. 2020, 5, e000458. [Google Scholar] [CrossRef]
- Salimiaghdam, N.; Singh, L.; Schneider, K.; Chwa, M.; Atilano, S.R.; Nalbandian, A.; Limb, G.A.; Kenney, M.C. Effects of fluoroquinolones and tetracyclines on mitochondria of human retinal MIO-M1 cells. Exp. Eye Res. 2022, 214, 108857. [Google Scholar] [CrossRef]
- Salimiaghdam, N.; Riazi-Esfahani, M.; Fukuhara, P.S.; Schneider, K.; Kenney, M.C. Age-related macular degeneration (AMD): A review on its epidemiology and risk factors. Open Ophthalmol. J. 2019, 13, 90–99. [Google Scholar] [CrossRef]
- Musiał-Kopiejka, M.; Polanowska, K.; Dobrowolski, D.; Krysik, K.; Wylęgała, E.; Grabarek, B.O.; Lyssek-Boroń, A. The Effectiveness of Brolucizumab and Aflibercept in Patients with Neovascular Age-Related Macular Degeneration. Int. J. Env. Res. Public Health 2022, 19, 2303. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Pavlis, J.M.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Hsu, T.; Woo, G.; Soe, K. Mitochondrial DNA variants mediate energy production and expression levels for CFH, C3 and EFEMP1 genes: Implications for age-related macular degeneration. PLoS ONE 2013, 8, e54339. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.E.; Schaier, E.; Brunner, S.M.; Eder, W.; Mayr, J.A.; Egger, S.F.; Nischler, C.; Oberkofler, H.; Reitsamer, H.A.; Patsch, W. Mitochondrial haplogroups and control region polymorphisms in age-related macular degeneration: A case-control study. PLoS ONE 2012, 7, e30874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Falatoonzadeh, P.; Ramirez, C.; Malik, D.; Tarek, M.; Cáceres-del-Carpio, J.; Nesburn, A.B.; Boyer, D.S. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: Insights into mitochondrial–nuclear interactions. Hum. Mol. Genet. 2014, 23, 3537–3551. [Google Scholar] [CrossRef] [Green Version]
- Dunn, K.; Aotaki-Keen, A.; Putkey, F.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.V.; Jazwinski, S.M. Nuclear gene expression changes due to mitochondrial dysfunction in ARPE-19 cells: Implications for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1765–1773. [Google Scholar] [CrossRef]
- Asiabi, P.; Ambroise, J.; Giachini, C.; Coccia, M.; Bearzatto, B.; Chiti, M.C.; Dolmans, M.-M.; Amorim, C. Assessing and validating housekeeping genes in normal, cancerous, and polycystic human ovaries. J. Assist. Reprod. Genet. 2020, 37, 2545–2553. [Google Scholar] [CrossRef]
- Etminan, M.; Forooghian, F.; Brophy, J.M.; Bird, S.T.; Maberley, D. Oral fluoroquinolones and the risk of retinal detachment. JAMA 2012, 307, 1414–1419. [Google Scholar]
- Nacht, J. Oral Fluoroquinolones and the Risk Of Uveitis. J. Emerg. Med. 2016, 5, 805–806. [Google Scholar] [CrossRef]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.-J.J.; Younis, H.; Boelsterli, U.A. Trovafloxacin, a fluoroquinolone antibiotic with hepatotoxic potential, causes mitochondrial peroxynitrite stress in a mouse model of underlying mitochondrial dysfunction. Chem. Biol. Interact. 2010, 188, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feher, J.; Kovacs, I.; Artico, M.; Cavallotti, C.; Papale, A.; Gabrieli, C.B. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol. Aging 2006, 27, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hangas, A.; Aasumets, K.; Kekäläinen, N.J.; Paloheinä, M.; Pohjoismäki, J.L.; Gerhold, J.M.; Goffart, S. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018, 46, 9625–9636. [Google Scholar] [CrossRef]
- Wagai, N.; Tawara, K. Possible direct role of reactive oxygens in the cause of cutaneous phototoxicity induced by five quinolones in mice. Arch. Toxicol. 1992, 66, 392–397. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Zang, L.; Zhang, Y.; Zhang, Y.; Wang, X.; Ai, W.; Ding, N.; Wang, H. Joint toxicity of fluoroquinolone and tetracycline antibiotics to zebrafish (Danio rerio) based on biochemical biomarkers and histopathological observation. J. Toxicol. Sci. 2017, 42, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Ahler, E.; Sullivan, W.J.; Cass, A.; Braas, D.; York, A.G.; Bensinger, S.J.; Graeber, T.G.; Christofk, H.R. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE 2013, 8, e64561. [Google Scholar]
- Glickman, R.D. Phototoxicity to the retina: Mechanisms of damage. Int. J. Toxicol. 2002, 21, 473–490. [Google Scholar] [CrossRef]
- Jones, C.N.; Miller, C.; Tenenbaum, A.; Spremulli, L.L.; Saada, A. Antibiotic effects on mitochondrial translation and in patients with mitochondrial translational defects. Mitochondrion 2009, 9, 429–437. [Google Scholar] [CrossRef]






| Cybrid | Haplogroup | Age | Sex | Ethnicity | Diagnosis |
|---|---|---|---|---|---|
| 16-188 | K | 90 | M | Caucasian | Dry AMD |
| 13-129 | K1a1b1a | 89 | M | Caucasian | Wet AMD |
| 16-187 | K2a2a1 | 82 | M | Caucasian | Dry AMD |
| 14-138 | U2e1a1 | 69 | M | Caucasian | Dry AMD |
| 17-200 | U | 69 | M | Caucasian | Dry AMD |
| 18-238 | U | 76 | F | Caucasian | Wet AMD |
| 14-136 | J2a1a1a2 | 77 | F | Caucasian | Wet AMD |
| 14-142 | J1c2g | 91 | F | Caucasian | Wet AMD |
| Symbol | Gene Name | GenBank Accession No. | Sigma Primer Sequences Or Qiagen Gene Globe ID | Function |
|---|---|---|---|---|
| CASP-3 | Caspase 3, apoptosis-related cysteine peptidase | NM_004346 NM_032991 | QT00023947 | Encodes protein as a cysteine–aspartic acid protease that plays a central role in the execution phase of cell apoptosis. |
| CASP-7 | Caspase 7, apoptosis-related cysteine peptidase | NM_145248, XM_006725153, XM_006725154, XM_005268295, XM_006725155, XM_005268294, XM_006719962 | QT00003549 | This gene encodes a member of the cysteine–aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution phase of cell apoptosis. |
| CASP-9 | Caspase 9, apoptosis-related cysteine peptidase | NM_001229 NM_032996 | QT00036267 | Encodes a member of the cysteine–aspartic acid protease (caspase) family, which is involved in the execution phase of cell apoptosis. |
| IL-6 | Interleukin 6 | NM_000600 | FH1-5′-GCAGAAAAAGGCAAAGAATG-3′ RH1-5′-CTACATTTGCCGAAGAGC-3′ | This gene encodes a cytokine that functions in inflammation and the maturation of B cells. In addition, the encoded protein has been shown to be an endogenous pyrogen capable of inducing fever in people with autoimmune diseases or infections. |
| IL-33 | Interleukin 33 | NM_033439 NM_001199640 NM_001127180 | FH1-5′-CCAGAAGTCTTTTGTAGG-3′ RH1-5′-GCTGGGAAATAAGGTGTT-3′ | The protein encoded by this gene is a cytokine that binds to the IL1RL1/ST2 receptor. The encoded protein is involved in the maturation of Th2 cells and the activation of mast cells, basophils, eosinophils and natural killer cells. |
| TGF-β1 | Transforming growth factor beta-1-like | NM_003238 | FH1-5′-AACCCACAACGAAATCTATG-3′ RH1-5′-CTTTTAACTTGAGCCTCA-GC-3′ | This gene is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation and apoptosis. |
| SOD1 | Superoxide dismutase 1 | NM_000454 | QT01671551 | This gene is a member of the iron/manganese superoxide dismutase family. The protein encoded by this gene is a soluble cytoplasmic protein, acting as a homodimer to convert naturally occurring but harmful superoxide radicals to molecular oxygen and hydrogen peroxide. |
| SOD2 | Superoxide dismutase 2 | NM_000636 | FH1-5′-ATCTACCCTAATGATCCCAG-3′ RH1-5′-AGGACCTTATAGGGTTTTCAG-3′ | This gene encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. |
| SOD3 | Superoxide dismutase 3 | NM-003102 | QT01664327 | This gene encodes a member of the superoxide dismutase (SOD) protein family, which catalyzes the conversion of superoxide radicals into hydrogen peroxide and oxygen, effective in protection of the brain, lungs and other tissues from oxidative stress. |
| GPX3 | Glutathione peroxidase 3 | NM_002084 | FH1-5′-GCAACCAATTTGGAAAACAG-3′ RH1-5′-CTCAAAGAGCTGGAAATTAGG-3′ | The protein encoded by this gene belongs to the glutathione peroxidase family, members of which catalyze the reduction of organic hydroperoxides and hydrogen peroxide (H2O2) by glutathione, and thereby protect cells against oxidative damage. Several isozymes of this gene family exist in vertebrates, which vary in cellular location and substrate specificity. |
| HPRT1 | Hypoxanthine Phosphoribosyl transferase 1 | NM_000194 | FH1-5′-ATAAGCCAGACTTTGTTGG-3′ RH1-5′-ATAGGACTCCAGATGTTTCC-3′ | The protein encoded by this gene is a transferase, which catalyzes conversion of hypoxanthine to inosine monophosphate and guanine to guanosine monophosphate via transfer of the 5-phosphoribosyl group from 5-phosphoribosyl 1-pyrophosphate. This enzyme plays a central role in the generation of purine nucleotides through the purine salvage pathway. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimiaghdam, N.; Singh, L.; Singh, M.K.; Chwa, M.; Atilano, S.R.; Mohtashami, Z.; Nesburn, A.B.; Kuppermann, B.D.; Lu, S.Y.; Kenney, M.C. Impacts of Bacteriostatic and Bactericidal Antibiotics on the Mitochondria of the Age-Related Macular Degeneration Cybrid Cell Lines. Biomolecules 2022, 12, 675. https://doi.org/10.3390/biom12050675
Salimiaghdam N, Singh L, Singh MK, Chwa M, Atilano SR, Mohtashami Z, Nesburn AB, Kuppermann BD, Lu SY, Kenney MC. Impacts of Bacteriostatic and Bactericidal Antibiotics on the Mitochondria of the Age-Related Macular Degeneration Cybrid Cell Lines. Biomolecules. 2022; 12(5):675. https://doi.org/10.3390/biom12050675
Chicago/Turabian StyleSalimiaghdam, Nasim, Lata Singh, Mithalesh K. Singh, Marilyn Chwa, Shari R. Atilano, Zahra Mohtashami, Anthony B. Nesburn, Baruch D. Kuppermann, Stephanie Y. Lu, and M. Cristina Kenney. 2022. "Impacts of Bacteriostatic and Bactericidal Antibiotics on the Mitochondria of the Age-Related Macular Degeneration Cybrid Cell Lines" Biomolecules 12, no. 5: 675. https://doi.org/10.3390/biom12050675
APA StyleSalimiaghdam, N., Singh, L., Singh, M. K., Chwa, M., Atilano, S. R., Mohtashami, Z., Nesburn, A. B., Kuppermann, B. D., Lu, S. Y., & Kenney, M. C. (2022). Impacts of Bacteriostatic and Bactericidal Antibiotics on the Mitochondria of the Age-Related Macular Degeneration Cybrid Cell Lines. Biomolecules, 12(5), 675. https://doi.org/10.3390/biom12050675