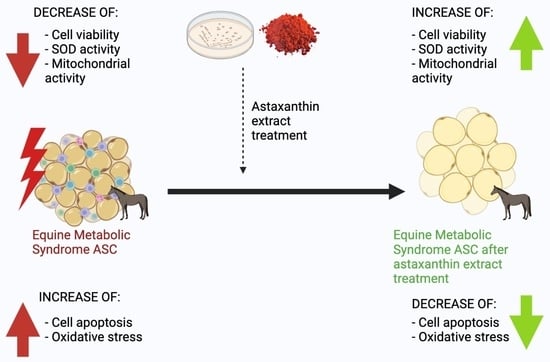
Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Biomass and Astaxanthin Extraction
2.2. Equine ASCs Cell Culture
2.3. Determination of Cell Viability and Proliferative Activity by TOX8 Assay
2.4. Bromodeoxyuridine (BrdU) Assay
2.5. Colony-Forming Unit-Fibroblast (CFU-fs) Assay
2.6. Flow Cytometric Analysis of Cell Viability and Apoptosis
2.7. Intracellular Reactive Oxygen Species Determination
2.8. Endogenous Antioxidant Activities Assays
2.9. Mitochondrial Membrane Potential Assay (MMP)
2.10. Mitochondrial Network Fluorescent Staining
2.11. Mitochondria Isolation for Transcriptomic Analysis
2.12. RNA Extraction and Real-Time Reverse Transcription PCR (qRT-PCR)
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Astaxanthin Improves Viability and Proliferation in EMS ASCs Affected Cells
3.2. Astaxanthin Reduced Cell Apoptosis in Equine ASC Cells Suffering from EMS
3.3. Astaxanthin Decreases Oxidative Stress in Equine EMS ASC Cells
3.4. Astaxanthin Enhances Mitochondrial Dynamics in EMS Affected ASC cells
3.5. Astaxanthin Supports the Transcription of Mitochondrial Metabolism Related Effectors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swarup, S.; Goyal, A.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal Models of Metabolic Syndrome: A Review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Gupta, V. Metabolic Syndrome: What Are the Risks for Humans? Biosci. Trends 2010, 4, 204–212. [Google Scholar] [PubMed]
- Grzesiak, J.; Marycz, K.; Czogala, J.; Wrzeszcz, K.; Nicpon, J. Comparison of Behavior, Morphology and Morphometry of Equine and Canine Adipose Derived Mesenchymal Stem Cells in Culture. Int. J. Morphol. 2011, 29, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM Consensus Statement on Equine Metabolic Syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef]
- Bourebaba, L.; Kornicka-Garbowska, K.; Al Naem, M.; Röcken, M.; Łyczko, J.; Marycz, K. MSI-1436 Improves EMS Adipose Derived Progenitor Stem Cells in the Course of Adipogenic Differentiation through Modulation of ER Stress, Apoptosis, and Oxidative Stress. Stem Cell Res. Ther. 2021, 12, 97. [Google Scholar] [CrossRef]
- Guo, S. Insulin Signaling, Resistance, and the Metabolic Syndrome: Insights from Mouse Models into Disease Mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Matulewicz, N.; Karczewska-Kupczewska, M. Insulinooporność a przewlekła reakcja zapalna. Postepy Hig. Med. Dosw. 2016, 70, 1245–1257. [Google Scholar]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [Green Version]
- Beale, E.G. Insulin Signaling and Insulin Resistance. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2013, 61, 11–14. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Grzesiak, J.; Wrzeszcz, K.; Golonka, P. Adipose Stem Cell Combined with Plasma-Based Implant Bone Tissue Differentiation in Vitro and in a Horse with a Phalanx Digitalis Distalis Fracture: A Case Report. Veterinární Medicína 2012, 57, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Śmieszek, A.; Szłapka, J. Macroautophagy and Selective Mitophagy Ameliorate Chondrogenic Differentiation Potential in Adipose Stem Cells of Equine Metabolic Syndrome: New Findings in the Field of Progenitor Cells Differentiation. Oxid. Med. Cell. Longev. 2016, 2016, 3718468. [Google Scholar] [CrossRef]
- Basinska, K.; Marycz, K.; Śmieszek, A.; Nicpoń, J. The Production and Distribution of IL-6 and TNF-α in Subcutaneous Adipose Tissue and Their Correlation with Serum Concentrations in Welsh Ponies with Equine Metabolic Syndrome. J. Vet. Sci. 2015, 16, 113. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic Review Series: Adipocyte Biology. Adipose Tissue Function and Plasticity Orchestrate Nutritional Adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Liu, M. Adipose Tissue in Control of Metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Reynolds, A.; Keen, J.A.; Fordham, T.; Morgan, R.A. Adipose Tissue Dysfunction in Obese Horses with Equine Metabolic Syndrome. Equine Vet. J. 2019, 51, 760–766. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–58. [Google Scholar] [CrossRef]
- Marycz, K.; Basinska, K.; Toker, N.Y.; Śmieszek, A.; Nicpoń, J. Atlarda Metabolik Sendrom (EMS) Sancısında, Adipoz Dokuda ve Periferal Kanda IL-6 ve TNF-α Nın Aktivitesi. Kafkas Univ. Vet. Fak. Derg. 2014, 20, 493–499. [Google Scholar] [CrossRef]
- Langin, D. Control of Fatty Acid and Glycerol Release in Adipose Tissue Lipolysis. Comptes Rendus Biologies 2006, 329, 598–607. [Google Scholar] [CrossRef]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered Lipid Metabolism and the Pathogenesis of Insulin Resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Kornicka, K.; Szłapka-Kosarzewska, J.; Śmieszek, A.; Marycz, K. 5-Azacytydine and Resveratrol Reverse Senescence and Ageing of Adipose Stem Cells via Modulation of Mitochondrial Dynamics and Autophagy. J. Cell. Mol. Med. 2019, 23, 237–259. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; El-Sabbagh, A.S.; Lukas, B.E.; Tanneberger, S.J.; Jiang, Y. Adipose Stem Cells in Obesity: Challenges and Opportunities. Biosci. Rep. 2020, 40, BSR20194076. [Google Scholar] [CrossRef]
- Schosserer, M.; Grillari, J.; Wolfrum, C.; Scheideler, M. Age-Induced Changes in White, Brite, and Brown Adipose Depots: A Mini-Review. Gerontology 2018, 64, 229–236. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive Oxygen Species, Cell Signaling, and Cell Injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Castro, J.P.; Grune, T.; Speckmann, B. The Two Faces of Reactive Oxygen Species (ROS) in Adipocyte Function and Dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C. Oxidative Stress. JPEN J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and Other Nutrients from Haematococcus Pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Grimmig, B.; Kim, S.-H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective Mechanisms of Astaxanthin: A Potential Therapeutic Role in Preserving Cognitive Function in Age and Neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, 2100252. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, E239. [Google Scholar] [CrossRef]
- Masoudi, A.; Dargahi, L.; Abbaszadeh, F.; Pourgholami, M.H.; Asgari, A.; Manoochehri, M.; Jorjani, M. Neuroprotective Effects of Astaxanthin in a Rat Model of Spinal Cord Injury. Behav. Brain Res. 2017, 329, 104–110. [Google Scholar] [CrossRef]
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E.; et al. Nanotechnology-Abetted Astaxanthin Formulations in Multimodel Therapeutic and Biomedical Applications. J. Med. Chem. 2022, 65, 2–36. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Protection against Oxidative Stress, Inflammation, and Apoptosis of High-Glucose-Exposed Proximal Tubular Epithelial Cells by Astaxanthin. J. Agric. Food Chem. 2009, 57, 8793–8797. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Astaxanthin for Its Use as a Novel Food in Food Supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef] [Green Version]
- Earnest, C.P.; Lupo, M.; White, K.M.; Church, T.S. Effect of Astaxanthin on Cycling Time Trial Performance. Int. J. Sports Med. 2011, 32, 882–888. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective Effects of Astaxanthin on Skin Deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wen, Y.; Li, S.; Zhang, P.; Wang, Y.; Wang, J.; Cao, K.; Du, L.; Wang, N.; Jie, Y. Benefits and Safety of Astaxanthin in the Treatment of Mild-To-Moderate Dry Eye Disease. Front. Nutr. 2022, 8, 796951. [Google Scholar] [CrossRef]
- Shatoor, A.S.; Al Humayed, S. Astaxanthin Ameliorates High-Fat Diet-Induced Cardiac Damage and Fibrosis by Upregulating and Activating SIRT1. Saudi J. Biol. Sci. 2021, 28, 7012–7021. [Google Scholar] [CrossRef]
- Murai, T.; Kawasumi, K.; Tominaga, K.; Okada, Y.; Kobayashi, M.; Arai, T. Effects of Astaxanthin Supplementation in Healthy and Obese Dogs. Vet. Med. Res. Rep. 2019, 10, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Marycz, K.; Toker, N.Y.; Grzesiak, J.; Wrzeszcz, K.; Golonka, P. The Therapeutic Effect of Autogenic Adipose Derived Stem Cells Combined with Autogenic Platelet Rich Plasma in Tendons Disorders Hi Horses in Vitro and in Vivo Research. J. Anim. Vet. Adv. 2012, 11, 4324–4331. [Google Scholar]
- Bourebaba, L.; Bedjou, F.; Röcken, M.; Marycz, K. Nortropane Alkaloids as Pharmacological Chaperones in the Rescue of Equine Adipose-Derived Mesenchymal Stromal Stem Cells Affected by Metabolic Syndrome through Mitochondrial Potentiation, Endoplasmic Reticulum Stress Mitigation and Insulin Resistance Alleviation. Stem Cell Res. Ther. 2019, 10, 178. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2021, 14, 107. [Google Scholar] [CrossRef]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Sotnikova, L.; Kruglov, A.; Krestinina, O. Astaxanthin Inhibits Mitochondrial Permeability Transition Pore Opening in Rat Heart Mitochondria. Antioxidants 2019, 8, 576. [Google Scholar] [CrossRef] [Green Version]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and Insulin Resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Weiss, C.; Kornicka-Grabowska, K.; Mularczyk, M.; Siwinska, N.; Marycz, K. Extracellular Microvesicles (MV’s) Isolated from 5-Azacytidine-and-Resveratrol-Treated Cells Improve Viability and Ameliorate Endoplasmic Reticulum Stress in Metabolic Syndrome Derived Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2020, 16, 1343–1355. [Google Scholar] [CrossRef]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.-X.; Zhou, H.-L.; Huang, C.-L.; You, C.-G.; Fang, Q.; Wu, P.; Wang, X.-G.; Han, C.-M. Astaxanthin Attenuates Early Acute Kidney Injury Following Severe Burns in Rats by Ameliorating Oxidative Stress and Mitochondrial-Related Apoptosis. Mar. Drugs 2015, 13, 2105–2123. [Google Scholar] [CrossRef] [Green Version]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta BBA Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Asano, T.; Katagiri, H.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Fujishiro, M.; Kushiyama, A.; Fukushima, Y.; et al. Oxidative Stress Induces Insulin Resistance by Activating the Nuclear Factor-?B Pathway and Disrupting Normal Subcellular Distribution of Phosphatidylinositol 3-Kinase. Diabetologia 2004, 47, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Peña-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Jose Tinahones, F.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative Stress Is Associated with the Number of Components of Metabolic Syndrome: LIPGENE Study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses. Stem Cells Int. 2018, 2018, 5340756. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a Molecular Therapeutic Target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and Anti-inflammatory Mechanisms of Action of Astaxanthin in Cardiovascular Diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Barros, M.P.; Pinto, E.; Colepicolo, P.; Pedersén, M. Astaxanthin and Peridinin Inhibit Oxidative Damage in Fe(2+)-Loaded Liposomes: Scavenging Oxyradicals or Changing Membrane Permeability? Biochem. Biophys. Res. Commun. 2001, 288, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Sun, C.; Hao, Q.; Cheng, J. Astaxanthin Ameliorates Cardiomyocyte Apoptosis after Coronary Microembolization by Inhibiting Oxidative Stress via Nrf2/HO-1 Pathway in Rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 341–348. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Irwin-Houston, J.M.; Weiss, C. Combination of Resveratrol and 5-Azacytydine Improves Osteogenesis of Metabolic Syndrome Mesenchymal Stem Cells. J. Cell. Mol. Med. 2018, 22, 4771–4793. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, E1137. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin Stimulates Mitochondrial Biogenesis in Insulin Resistant Muscle via Activation of AMPK Pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Krestinina, O.; Baburina, Y.; Krestinin, R.; Odinokova, I.; Fadeeva, I.; Sotnikova, L. Astaxanthin Prevents Mitochondrial Impairment Induced by Isoproterenol in Isolated Rat Heart Mitochondria. Antioxidants 2020, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Krestinina, O.; Baburina, Y.; Krestinin, R. Mitochondrion as a Target of Astaxanthin Therapy in Heart Failure. Int. J. Mol. Sci. 2021, 22, 7964. [Google Scholar] [CrossRef]
- Chen, Y.; Tie, S.; Zhang, X.; Zhang, L.; Tan, M. Preparation and Characterization of Glycosylated Protein Nanoparticles for Astaxanthin Mitochondria Targeting Delivery. Food Funct. 2021, 12, 7718–7727. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but Not Quercetin Preserves Mitochondrial Integrity and Function, Ameliorates Oxidative Stress, and Reduces Heat-Induced Skeletal Muscle Injury. J. Cell. Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, H.; Zhang, L.; Wu, B.; Zha, Z. Maintenance of Mitochondrial Function by Astaxanthin Protects against Bisphenol A-Induced Kidney Toxicity in Rats. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Bergman, O.; Ben-Shachar, D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) Deficits in Schizophrenia: Possible Interactions with Cellular Processes. Can. J. Psychiatry 2016, 61, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of Mitochondrial Oxidative Phosphorylation Supercomplexes and Mechanisms for Their Stabilisation. Dyn. Ultrastruct. Bioenerg. Membr. Compon. 2014, 1837, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Bourebaba, N.; Kornicka-Garbowska, K.; Marycz, K.; Bourebaba, L.; Kowalczuk, A. Laurus Nobilis Ethanolic Extract Attenuates Hyperglycemia and Hyperinsulinemia Induced Insulin Resistance in HepG2 Cell Line through the Reduction of Oxidative Stress and Improvement of Mitochondrial Biogenesis-Possible Implication in Pharmacotherapy. Mitochondrion 2021, 59, 190–213. [Google Scholar] [CrossRef]
- Sato, F.; Omura, T.; Ishimaru, M.; Endo, Y.; Murase, H.; Yamashita, E. Effects of Daily Astaxanthin and L-Carnitine Supplementation for Exercise-Induced Muscle Damage in Training Thoroughbred Horses. J. Equine Vet. Sci. 2015, 35, 836–842. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina Platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef] [Green Version]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin Ameliorates Features of Metabolic Syndrome in SHR/NDmcr-Cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-Diabetic Effects of Astaxanthin on an STZ-Induced Diabetic Model in Rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef]









| Gene | Primer | Sequence 5′–3′ | Amplicon Length (bp) | Accession No. |
|---|---|---|---|---|
| Parkin | F: R: | GTGCAGAGACCGTGGAGAAA GCTGCACTGTACCCTGAGTT | 294 | NM_013987.3 |
| Sod1 (Cu/Zn SOD) | F: R: | CATTCCATCATTGGCCGCAC GAGCGATCCCAATCACACCA | 130 | NW_001867397.1 |
| Sod2 (Mn SOD) | F: R: | GGACAAACCTGAGCCCCAAT TTGGACACCAGCCGATACAG | 125 | NW_001867408.1 |
| Pink1 | F: R: | GCTTGGGACCTCTCTTGGAT CGAAGCCATCTTGAACACAA | 142 | NM_032409.3 |
| Casp9 | F: R: | CAGGCCCCATATGATCGAGG CTGGCCTGTGTCCTCTAAGC | 142 | NM_032996.3 |
| Casp3 | F: R: | GGCAGACTTCCTGTATGCGT CCATGGCTACCTTGCGGTTA | 167 | XM_023630401.1 |
| Bcl-2 | F: R: | ATCGCCCTGTGGATGACTGAG CAGCCAGGAGAAATCAAACAGAGG | 129 | NM_000633.2 |
| p21 | F: R: | AGAAGAGGCTGGTGGCTATTT CCCGCCATTAGCGCATCAC | 169 | NM_001220777.1 |
| p53 | F: R: | AGATAGCGATGGTCTGGC TTGGGCAGTGCTCGCTTAGT | 381 | NM_001126118.1 |
| Casp8 | F: R: | ACTGTGATGTTGCTGGGACT CTTTCTCCTGGTGCATCTATCG | 177 | XM_001496753.4 |
| Bax | F: R: | ACCAAGAAGCTGAGCGAGTGTC ACAAAGATGGTCACGGTCTGCC | 356 | XM_011527191.1 |
| Mfn1 | F: R: | GTTGCCGGGTGATAGTTGGA TGCCACCTTCATGTGTCTCC | 146 | NM_033540.3 |
| OPA1 | F:R: | CTTCTCTTGTTAGGTTCACCTGG TGTAAGAGAATGAGCTCACCAAG | 110 | XM_003363363.4 |
| GAPDH | F: R: | GTCAGTGGTGGACCTGACCT CACCACCCTGTTGCTGTAGC | 256 | NM_001357943.2 |
| Wnt3 | F: R: | CACCTGCAAGTAGGGAGCCA GCTTCCCAGAGGACTTCGGT | 80 | XM_014739584.2 |
| NDUFA9 | F: R: | TTGGTATTCAGGCCACACCC GCTGGCTTCACGTCTTCAAC | 103 | XM_001494601.4 |
| UQCRC2 | F: R: | TGCTTCGTCTTGCATCCAGT AACTCCGGTGACGTGGTAAC | 193 | XM_001494381.5 |
| COX4I1 | F: R: | GAATAGGGGCACGAACGAGT GCCACCCACTCCTCTTCAAA | 138 | XM_023637444.1 |
| COX4I2 | F: R: | CCCCACCCCAGATGTTCT CGTGGTAGTTGGTGTAGGG | 135 | XM_005604417.3 |
| OXA1L | F: R: | GACCTAGAAACCGTGGGACG GGAAGATCACTTGGCTCCCC | 105 | XM_008528958.1 |
| MRPL24 | F: R: | ATGATCCCTAGCGAAGCACC TGTAGAGACTCGTACCCGCT | 123 | XM_001500466.4 |
| MTERF4 | F: R: | CGCCACCTCCGTGCTATG CCCAAATGAGGGGCATCAGG | 147 | XM_023644068.1 |
| PUSL1 | F: R: | TCAGCCACTTCCAGGACCTA AGCCACATCCAAGCTGTCTG | 120 | XM_023636046.1 |
| TFAM | F: R: | ATGATGGCTTTGAGTCCAGG CTAGATGATGGCGGGAGACTT | 154 | XM_023643450.1 |
| Antibody | Dilution | Catalog No. |
|---|---|---|
| PINK 1 | 1:1000 | Biorbyt, orb331223 |
| MFF | 1:1000 | Biorbyt, orb325479 |
| β-Actin | 1:1000 | Sigma Aldrich, a2066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mularczyk, M.; Bourebaba, N.; Marycz, K.; Bourebaba, L. Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis. Biomolecules 2022, 12, 1039. https://doi.org/10.3390/biom12081039
Mularczyk M, Bourebaba N, Marycz K, Bourebaba L. Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis. Biomolecules. 2022; 12(8):1039. https://doi.org/10.3390/biom12081039
Chicago/Turabian StyleMularczyk, Malwina, Nabila Bourebaba, Krzysztof Marycz, and Lynda Bourebaba. 2022. "Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis" Biomolecules 12, no. 8: 1039. https://doi.org/10.3390/biom12081039
APA StyleMularczyk, M., Bourebaba, N., Marycz, K., & Bourebaba, L. (2022). Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis. Biomolecules, 12(8), 1039. https://doi.org/10.3390/biom12081039