Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review
Abstract
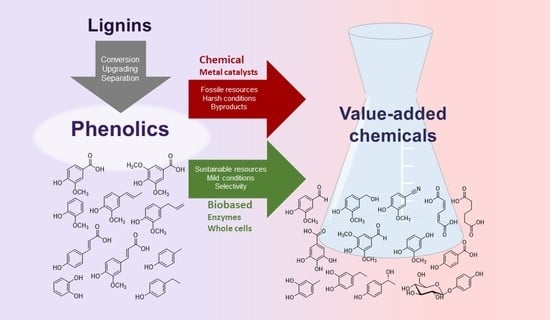
:1. Introduction
2. Vanillin
Reduction of Vanillin to Vanillyl Alcohol
3. Vanillic Acid
3.1. Reduction of Vanillic Acid to Vanillin
3.2. Cascade Reactions from Vanillic Acid to Vanillaldoxime and Vanillonitrile
3.3. Cascade Reaction from Vanillic Acid or Vanillin to Methoxyhydroquinone
3.4. Cascade Reaction from Vanillic Acid to Gallic Acid
4. Syringic Acid
4.1. Reduction of Syringic Acid to Syringaldehyde
5. Ferulic Acid
5.1. Transformation of Ferulic Acid to Vanillin
5.2. Transformation of Ferulic Acid to Vanillic Acid
6. p-Coumaric Acid
6.1. Transformation of p-Coumaric Acid to p-Hydroxybenzoic Acid
6.1.1. Transformation of p-Hydroxybenzoic Acid to Arbutin
7. Guaiacol and Alkyl Guaiacols
Enzymatic Cascades from Guaiacol to cis,cis-Muconic Acid and Adipic Acid
8. Eugenol and Isoeugenol
Oxidation of (Iso)Eugenol to Vanillin
9. Alkylphenols
9.1. O-Hydroxylation of Alkylphenols to Catechols
9.2. Oxidation of 4-Ethylphenol to Chiral Secondary Alcohol
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Wolf, M.E.; Hinchen, D.J.; DuBois, J.L.; McGeehan, J.E.; Eltis, L.D. Cytochromes P450 in the biocatalytic valorization of lignin. Curr. Opin. Biotechnol. 2022, 73, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Koelewijn, S.F.; Renders, T.; Van den Bossche, G.; Vangeel, T.; Schutyser, W.; Sels, B.F. Catalytic strategies towards lignin-derived chemicals. Top. Curr. Chem. 2018, 376, 36. [Google Scholar] [CrossRef] [PubMed]
- Damm, T.; Grande, P.M.; Jablonowski, N.D.; Thiele, B.; Disko, U.; Mann, U.; Schurr, U.; Leitner, W.; Usadel, B.; Domínguez de María, P.; et al. OrganoCat pretreatment of perennial plants: Synergies between a biogenic fractionation and valuable feedstocks. Bioresour. Technol. 2017, 244, 889–896. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhu, L.L.; Wallraf, A.M.; Rauber, C.; Grande, P.M.; Anders, N.; Gertler, C.; Werner, B.; Klankermayer, J.; Leitner, W.; et al. Depolymerization of laccase-oxidized lignin in aqueous alkaline solution at 37 degrees C. ACS Sustain. Chem. Eng. 2019, 7, 11150–11156. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin depolymerization and biotransformation to industrially important chemicals/biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Pasma, S.A.; Daik, R.; Ramli, S.; Maskat, M.Y.; Zulfakar, M.H. Enzymatic degradation of lignin extracted from oil palm empty fruit bunch using laccase and cutinase. Bioresources 2019, 14, 8879–8891. [Google Scholar] [CrossRef]
- Broglia, F.; Rimoldi, L.; Meroni, D.; De Vecchi, S.; Morbidelli, M.; Ardizzone, S. Guaiacol hydrodeoxygenation as a model for lignin upgrading. Role of the support surface features on Ni-based alumina-silica catalysts. Fuel 2019, 243, 501–508. [Google Scholar] [CrossRef]
- Liu, X.H.; Jia, W.D.; Xu, G.Y.; Zhang, Y.; Fu, Y. Selective hydrodeoxygenation of lignin-derived phenols to cyclohexanols over Co-based catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8594–8601. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dhepe, P.L. Upgrading lignin derived monomers over basic supported metal catalysts. Fuel 2021, 306, 121588. [Google Scholar] [CrossRef]
- Bomont, L.; Alda-Onggar, M.; Fedorov, V.; Aho, A.; Peltonen, J.; Eranen, K.; Peurla, M.; Kumar, N.; Warna, J.; Russo, V.; et al. Production of cycloalkanes in hydrodeoxygenation of isoeugenol over Pt- and Ir-modified bifunctional catalysts. Eur. J. Inorg. Chem. 2018, 2841–2854. [Google Scholar] [CrossRef]
- Jung, B.K.; Lee, J.; Ha, J.M.; Lee, H.; Suh, D.J.; Jun, C.H.; Jae, J. Effective hydrodeoxygenation of lignin-derived phenols using bimetallic RuRe catalysts: Effect of carbon supports. Catal. Today 2018, 303, 191–199. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, P.Y.; Xie, S.X.; Sun, S. Recent advancements and challenges in lignin valorization: Green routes towards sustainable bioproducts. Molecules 2022, 27, 6055. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Biotransformation of lignin: Mechanisms, applications and future work. Biotechnol. Progr. 2020, 36, e2922. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, Y.Q.; Xue, L.; Ma, F.Y.; Dai, S.Y.; Xie, S.X. Microbial lignin valorization through depolymerization to aromatics conversion. Trends Biotechnol. 2022, 40, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Li, B.Z.; Yuan, J.S.; Yuan, Y.J. Creative biological lignin conversion routes toward lignin valorization. Trends Biotechnol. 2022, 40, 1550–1566. [Google Scholar] [CrossRef]
- Haldar, D.; Dey, P.; Thomas, J.; Singhania, R.R.; Patel, A.K. One pot bioprocessing in lignocellulosic biorefinery: A review. Bioresour. Technol. 2022, 365, 128180. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wan, Z.; Han, Y.; Jiao, Y.; Li, Z.H.; Fu, P.; Li, N.; Zhang, A.D.; Yi, W.M. A review on lignin waste valorization by catalytic pyrolysis: Catalyst, reaction system, and industrial symbiosis mode. J. Environ. Chem. Eng. 2023, 11, 109113. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Liu, L.W.; Zhao, S.; Huang, Z.S.; Li, C.T.; Jiang, S.X.; Li, Q.; Gu, P.F. Biosynthesis of vanillin by different microorganisms: A review. World J. Microbiol. Biotechnol. 2022, 38, 40. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef]
- Grajales-Hernández, D.A.; Armendáriz Ruiz, M.A.A.; Contreras-Jácquez, V.; Mateos-Díaz, J.C. Biotransformation of phenolic acids from byproducts using heterogeneous biocatalysts: One more step toward a circular economy. Curr. Opin. Green Sustain. Chem. 2021, 32, 100550. [Google Scholar] [CrossRef]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of plant-derived phenolic acids. Biotechnol. J. 2018, 13, 1700632. [Google Scholar] [CrossRef]
- Ullah, I.; Chen, Z.B.; Xie, Y.X.; Khan, S.S.; Singh, S.; Yu, C.Y.; Cheng, G. Recent advances in biological activities of lignin and emerging biomedical applications: A short review. Int. J. Biol. Macromol. 2022, 208, 819–832. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, Y.T.; Cheng, Y.; Sun, H.L.; Bai, S.W.; Li, C.Y. Identifying environmental hotspots and improvement strategies of vanillin production with life cycle assessment. Sci. Total Environ. 2021, 769, 144771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, X.N.; Whelton, A.J.; Abu-Omar, M.M. Renewable epoxy thermosets from fully lignin-derived triphenols. ACS Sustain. Chem. Eng. 2018, 6, 7600–7608. [Google Scholar] [CrossRef]
- Yu, A.Z.; Serum, E.M.; Renner, A.C.; Sahouani, J.M.; Sibi, M.P.; Webster, D.C. Renewable reactive diluents as practical styrene replacements in biobased vinyl ester thermosets. ACS Sustain. Chem. Eng. 2018, 6, 12586–12592. [Google Scholar] [CrossRef]
- Wang, B.B.; Ma, S.Q.; Xu, X.W.; Li, Q.; Yu, T.; Wang, S.; Yan, S.F.; Liu, Y.L.; Zhu, J. High-performance, biobased, degradable polyurethane thermoset and its application in readily recyclable carbon fiber composites. ACS Sustain. Chem. Eng. 2020, 8, 11162–11170. [Google Scholar] [CrossRef]
- Luo, D.X.; Guo, S.X.; He, F.; Chen, S.H.; Dai, A.; Zhang, R.F.; Wu, J. Design, synthesis, and bioactivity of α-ketoamide derivatives bearing a vanillin skeleton for crop diseases. J. Agric. Food Chem. 2020, 68, 7226–7234. [Google Scholar] [CrossRef]
- Scipioni, M.; Kay, G.; Megson, I.L.; Lin, P.K.T. Synthesis of novel vanillin derivatives: Novel multi-targeted scaffold ligands against Alzheimer’s disease. MedChemComm 2019, 10, 764–777. [Google Scholar] [CrossRef]
- Naz, H.; Tarique, M.; Khan, P.; Luqman, S.; Ahamad, S.; Islam, A.; Ahmad, F.; Hassan, M.I. Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol. Cell. Biochem. 2018, 438, 35–45. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Younis, N.N.; Shaheen, M.A.; Elseweidy, M.M. The synergistic effect between vanillin and doxorubicin in ehrlich ascites carcinoma solid tumor and MCF-7 human breast cancer cell line. Pathol. Res. Pract. 2016, 212, 767–777. [Google Scholar] [CrossRef]
- Ronnander, J.; Ljunggren, J.; Hedenstrom, E.; Wright, S.A.I. Biotransformation of vanillin into vanillyl alcohol by a novel strain of Cystobasidium laryngis isolated from decaying wood. AMB Express 2018, 8, 137. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Gnanasekar, P.; Nair, S.S.; Yi, S.L.; Yan, N. Curing behavior and thermomechanical performance of bioepoxy resin synthesized from vanillyl alcohol: Effects of the curing agent. Polymers 2021, 13, 2891. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Hao, Y.H.; Zhou, Y.N.; Han, Z.Y.; Xie, C.; Su, W.Y.; Hao, H.X. Solubility and thermodynamic properties of vanillyl alcohol in some pure solvents. J. Chem. Thermodyn. 2017, 106, 276–284. [Google Scholar] [CrossRef]
- Guo, X.J.; Gao, G.; Remon, J.; Ma, Y.; Jiang, Z.C.; Shi, B.; Tsang, D.C.W. Selective hydrogenation of vanillin to vanillyl alcohol over Pd, Pt, and Au catalysts supported on an advanced nitrogen-containing carbon material produced from food waste. Chem. Eng. J. 2022, 440, 135885. [Google Scholar] [CrossRef]
- Wright, S.A.I.; de Felice, D.V.; Ianiri, G.; Pinedo-Rivilla, C.; De Curtis, F.; Castoria, R. Two rapid assays for screening of patulin biodegradation. Int. J. Environ. Sci. Technol. 2014, 11, 1387–1398. [Google Scholar] [CrossRef]
- Rocha, I.L.D.; Lopes, A.M.D.; Ventura, S.P.M.; Coutinho, J.A.P. Selective separation of vanillic acid from other lignin-derived monomers using centrifugal partition chromatography: The effect of pH. ACS Sustain. Chem. Eng. 2022, 10, 4913–4921. [Google Scholar] [CrossRef] [PubMed]
- Kuhire, S.S.; Ichake, A.B.; Grau, E.; Cramail, H.; Wadgaonkar, P.P. Synthesis and characterization of partially bio-based polyimides based on biphenylene-containing diisocyanate derived from vanillic acid. Eur. Polym. J. 2018, 109, 257–264. [Google Scholar] [CrossRef]
- Zhang, S.L.; Cheng, Z.Z.; Zeng, S.; Li, G.Y.; Xiong, J.; Ding, L.; Gauthier, M. Synthesis and characterization of renewable polyesters based on vanillic acid. J. Appl. Polym. Sci. 2020, 137, e49189. [Google Scholar] [CrossRef]
- Kasmi, N.; Papadopoulos, L.; Chebbi, Y.; Papageorgiou, G.Z.; Bikiaris, D.N. Effective and facile solvent-free synthesis route to novel biobased monomers from vanillic acid: Structure-thermal property relationships of sustainable polyesters. Polym. Degrad. Stab. 2020, 181, 109315. [Google Scholar] [CrossRef]
- Zhu, H.C.; Yang, J.J.; Wu, M.Y.; Wu, Q.Y.; Liu, J.Y.; Zhang, J.A. Vanillic acid as a new skeleton for formulating a biobased plasticizer. ACS Sustain. Chem. Eng. 2021, 9, 15322–15330. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Horvat, M.; Fiume, G.; Fritsche, S.; Winkler, M. Discovery of carboxylic acid reductase (CAR) from Thermothelomyces thermophila and its evaluation for vanillin synthesis. J. Biotechnol. 2019, 304, 44–51. [Google Scholar] [CrossRef]
- Kramer, L.; Le, X.; Hankore, E.D.; Wilson, M.A.; Guo, J.T.; Niu, W. Engineering and characterization of hybrid carboxylic acid reductases. J. Biotechnol. 2019, 304, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Schwendenwein, D.; Fiume, G.; Weber, H.; Rudroff, F.; Winkler, M. Selective enzymatic transformation to aldehydes in vivo by fungal carboxylate reductase from Neurospora crassa. Adv. Synth. Catal. 2016, 358, 3414–3421. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.S.; Oh, J.; Joo, J.C.; Yeon, Y.J. A highly active carboxylic acid reductase from Mycobacterium abscessus for biocatalytic reduction of vanillic acid to vanillin. Biochem. Eng. J. 2020, 161, 107683. [Google Scholar] [CrossRef]
- Strohmeier, G.A.; Eiteljorg, E.I.C.; Schwarz, A.; Winkler, M. Enzymatic one-step reduction of carboxylates to aldehydes with cell-free regeneration of ATP and NADPH. Chem.-Eur. J. 2019, 25, 6119–6123. [Google Scholar] [CrossRef]
- Winkler, M.; Horvat, M.; Schiefer, A.; Weilch, V.; Rudroff, F.; Pátek, M.; Martínková, L. Organic acid to nitrile: A chemoenzymatic three-step route. Adv. Synth. Catal. 2023, 365, 37–42. [Google Scholar] [CrossRef]
- Hinzmann, A.; Betke, T.; Asano, Y.; Groger, H. Synthetic processes toward nitriles without the use of cyanide: A biocatalytic concept based on dehydration of aldoximes in water. Chemistry 2021, 27, 5313–5321. [Google Scholar] [CrossRef]
- Lubbers, R.J.M.; Dilokpimol, A.; Nousiainen, P.A.; Visser, J.; Bruijnincx, P.C.A.; de Vries, R.P.; Cioc, R.C. Vanillic acid and methoxyhydroquinone production from guaiacyl units and related aromatic compounds using Aspergillus niger cell factories. Microb. Cell Fact. 2021, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, W.; Sahin, M.; Nothdurft, P.; Mourad, E.; Fruhwirt, P.; Riess, G.; Schmalegger, M.; Gescheidt-Demner, G.; Fischer, R.; Freunberger, S.; et al. 2-Methoxyhydroquinone from vanillin as bio-based active material for redox-flow batteries. Abstr. Pap. Am. Chem. Soc. 2019, 257, Meeting Abstract 397. [Google Scholar]
- Schlemmer, W.; Nothdurft, P.; Petzold, A.; Riess, G.; Fruhwirt, P.; Schmallegger, M.; Gescheidt-Demner, G.; Fischer, R.; Freunberger, S.A.; Kern, W.; et al. 2-Methoxyhydroquinone from vanillin for aqueous redox-flow batteries. Angew. Chem. Int. Ed 2020, 59, 22943–22946. [Google Scholar] [CrossRef]
- Cai, C.G.; Xu, Z.X.; Zhou, H.R.; Chen, S.T.; Jin, M.J. Valorization of lignin components into gallate by integrated biological hydroxylation, O-demethylation, and aryl side-chain oxidation. Sci. Adv. 2021, 7, abg4585. [Google Scholar] [CrossRef]
- Bonner, I.J.; Thompson, D.N.; Plummer, M.; Dee, M.; Tumuluru, J.S.; Pace, D.; Teymouri, F.; Campbell, T.; Bals, B. Impact of ammonia fiber expansion (AFEX) pretreatment on energy consumption during drying, grinding, and pelletization of corn stover. Dry. Technol. 2016, 34, 1319–1329. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef]
- Cheemanapalli, S.; Mopuri, R.; Golla, R.; Anuradha, C.M.; Chitta, S.K. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, J.; Yeon, Y.J. Biocatalytic valorization of lignin subunit: Screening a carboxylic acid reductase with high substrate preference to syringyl functional group. Enzym. Microb. Technol. 2022, 161, 110099. [Google Scholar] [CrossRef] [PubMed]
- Janvier, M.; Hollande, L.; Jaufurally, A.S.; Pernes, M.; Menard, R.; Grimaldi, M.; Beaugrand, J.; Balaguer, P.; Ducrot, P.H.; Allais, F. Syringaresinol: A renewable and safer alternative to bisphenol A for epoxy-amine resins. ChemSusChem 2017, 10, 738–746. [Google Scholar] [CrossRef]
- Wu, J.Y.; Fu, Y.S.; Lin, K.H.; Huang, X.; Chen, Y.J.; Lai, D.; Kang, N.; Huang, L.Y.; Weng, C.F. A narrative review: The pharmaceutical evolution of phenolic syringaldehyde. Biomed. Pharmacother. 2022, 153, 113339. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, W.K.; Wu, Q.C.; Yang, H.J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ludek, S.; Wawrzynczak, A.; Nowak, I.; Feliczak-Guzik, A. Synthesis of lipid nanoparticles incorporated with Ferula assa-foetida L. extract. Cosmetics 2022, 9, 129. [Google Scholar] [CrossRef]
- Dong, X.Y.; Huang, R. Ferulic acid: An extraordinarily neuroprotective phenolic acid with anti-depressive properties. Phytomedicine 2022, 105, 154355. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gulli, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent advances in the neuroprotective properties of ferulic acid in Alzheimer’s disease: A narrative review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef]
- Babbar, R.; Dhiman, S.; Grover, R.; Kaur, A.; Arora, S. A comprehensive review on therapeutic applications of ferulic acid and its novel analogues: A brief literature. Mini-Rev. Med. Chem. 2021, 21, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Volgger, G.; Wagner, A.O.; Zhang, D.C.; Poyntner, C. Biodegradation of lignin monomers and bioconversion of ferulic acid to vanillic acid by Paraburkholderia aromaticivorans AR20-38 isolated from Alpine forest soil. Appl. Microbiol. Biotechnol. 2021, 105, 2967–2977. [Google Scholar] [CrossRef]
- Zippilli, C.; Bartolome, M.J.; Hilberath, T.; Botta, L.; Hollmann, F.; Saladino, R. A photochemoenzymatic Hunsdiecker-Borodin-type halodecarboxylation of ferulic acid. ChemBioChem 2022, 23, e202200367. [Google Scholar] [CrossRef]
- Liberato, M.V.; Araujo, J.N.; Sodre, V.; Goncalves, T.A.; Vilela, N.; Moraes, E.C.; Garcia, W.; Squina, F.M. The structure of a prokaryotic feruloyl-CoA hydratase-lyase from a lignin-degrading consortium with high oligomerization stability under extreme pHs. BBA-Proteins Proteom. 2020, 1868, 140344. [Google Scholar] [CrossRef]
- Chen, Q.H.; Xie, D.T.; Qiang, S.; Hu, C.Y.; Meng, Y.H. Developing efficient vanillin biosynthesis system by regulating feruloyl-CoA synthetase and enoyl-CoA hydratase enzymes. Appl. Microbiol. Biotechnol. 2022, 106, 247–259. [Google Scholar] [CrossRef]
- Dippe, M.; Bauer, A.K.; Porzel, A.; Funke, E.; Müller, A.O.; Schmidt, J.; Beier, M.; Wessjohann, L.A. Coenzyme A-conjugated cinnamic acids-enzymatic synthesis of a CoA-ester library and application in biocatalytic cascades to vanillin derivatives. Adv. Synth. Catal. 2019, 361, 5346–5350. [Google Scholar] [CrossRef]
- Yao, X.Y.; Lv, Y.M.; Yu, H.L.; Cao, H.; Wang, L.Y.; Wen, B.T.; Gu, T.Y.; Wang, F.Z.; Sun, L.C.; Xin, F.J. Site-directed mutagenesis of coenzyme-independent carotenoid oxygenase CSO2 to enhance the enzymatic synthesis of vanillin. Appl. Microbiol. Biotechnol. 2020, 104, 3897–3907. [Google Scholar] [CrossRef]
- Saito, T.; Aono, R.; Furuya, T.; Kino, K. Efficient and long-term vanillin production from 4-vinylguaiacol using immobilized whole cells expressing Cso2 protein. J. Biosci. Bioeng. 2020, 130, 260–264. [Google Scholar] [CrossRef]
- Furuya, T.; Kuroiwa, M.; Kino, K. Biotechnological production of vanillin using immobilized enzymes. J. Biotechnol. 2017, 243, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, J.; Sharma, P.; Tomar, G.S.; Singh, S.; Grover, M.; Nain, L. One-pot microbial bioconversion of wheat bran ferulic acid to biovanillin. 3 Biotech 2021, 11, 462. [Google Scholar] [CrossRef]
- Valerio, R.; Bernardino, A.R.S.; Torres, C.A.V.; Brazinha, C.; Tavares, M.L.; Crespo, J.G.; Reis, M.A.M. Feeding strategies to optimize vanillin production by Amycolatopsis sp. ATCC 39116. Bioprocess. Biosyst. Eng. 2021, 44, 737–747. [Google Scholar] [CrossRef]
- Chakraborty, D.; Selvam, A.; Kaur, B.; Wong, J.W.C.; Karthikeyan, O.P. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) for bioconversion of agrowaste to vanillin. Appl. Microbiol. Biotechnol. 2017, 101, 5615–5626. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Yeoh, J.W.; Jayaraman, S.; Tan, S.G.D.; Jayaraman, P.; Holowko, M.B.; Zhang, J.Y.; Kang, C.W.; Leo, H.L.; Poh, C.L. A model-driven approach towards rational microbial bioprocess optimization. Biotechnol. Bioeng. 2021, 118, 305–318. [Google Scholar] [CrossRef]
- Luziatelli, F.; Brunetti, L.; Ficca, A.G.; Ruzzi, M. Maximizing the efficiency of vanillin production by biocatalyst enhancement and process optimization. Front. Bioeng. Biotechnol. 2019, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Kim, E.J.; Jung, E.; Kazlauskas, R.J.; Choi, K.Y.; Kim, B.G. Production of p-hydroxybenzoic acid from p-coumaric acid by Burkholderia glumae BGR1. Biotechnol. Bioeng. 2016, 113, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, J.; Ye, W.X.; Liao, K.S.; Wang, Z.S.; Song, X.F.; Qiao, M.Q. Synthetic biology-driven microbial production of resveratrol: Advances and perspectives. Front. Bioeng. Biotechnol. 2022, 10, 833920. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Yin, H.; Bi, H.P.; Zhuang, Y.B.; Liu, T.; Ma, Y.H. De novo biosynthesis of Gastrodin in Escherichia coli. Metab. Eng. 2016, 35, 138–147. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.L.; Peng, M.; Meng, H.Y.; Ma, H.B.; Cai, P.P.; Xu, Y.; Zhao, Q.; Si, G.M. A review on central nervous system effects of gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Qian, L.C.; Yan, S.H.; Li, Y.Z.; Wu, L.H.; Zheng, Y.W.; Wang, Y.X.; Fang, Z.Y. The effects of gastrodin injection on hypertension A systematic review and meta-analysis. Medicine 2020, 99, 20936. [Google Scholar] [CrossRef]
- Jurica, K.; Gobin, I.; Kremer, D.; Cepo, D.V.; Grubesic, R.J.; Karaconji, I.B.; Kosalec, I. Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Shang, Y.Z.; Wei, W.P.; Zhang, P.; Ye, B.C. Engineering Yarrowia lipolytica for enhanced production of arbutin. J. Agric. Food Chem. 2020, 68, 1364–1372. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, S.; Lee, H.; Kurisingal, J.F.; Han, S.H.; Kim, Y.H.; An, K. Complete utilization of waste lignin: Preparation of lignin-derived carbon supports and conversion of lignin-derived guaiacol to nylon precursors. Catal. Sci. Technol. 2022, 12, 5021–5031. [Google Scholar] [CrossRef]
- Barton, N.; Horbal, L.; Starck, S.; Kohlstedt, M.; Luzhetskyy, A.; Wittmann, C. Enabling the valorization of guaiacol-based lignin: Integrated chemical and biochemical production of cis,cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab. Eng. 2018, 45, 200–210. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, M.H.; Zhao, J.P.; Xia, H.H.; Xu, J.M.; Tan, W.H.; Jiang, J.C. Continuous steam-assisted low-temperature pyrolysis of alkali lignin and selective production of guaiacol components in a fixed-bed reactor. Energy Fuels 2019, 33, 8694–8701. [Google Scholar] [CrossRef]
- Shen, X.J.; Meng, Q.L.; Mei, Q.Q.; Liu, H.Z.; Yan, J.; Song, J.L.; Tan, D.X.; Chen, B.F.; Zhang, Z.R.; Yang, G.Y.; et al. Selective catalytic transformation of lignin with guaiacol as the only liquid product. Chem. Sci. 2020, 11, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liao, Y.H.; Bomon, J.; Tian, G.L.; Bai, S.T.; Van Aelst, K.; Zhang, Q.; Vermandel, W.; Wambacq, B.; Maes, B.U.W.; et al. Lignin-first monomers to catechol: Rational cleavage of C-O and C-C bonds over zeolites. ChemSusChem 2022, 15, e202102248. [Google Scholar] [CrossRef]
- Wang, A.R.; Dayo, A.Q.; Zu, L.W.; Xu, Y.L.; Lv, D.; Song, S.; Tang, T.; Liu, W.B.; Wang, J.; Gao, B.C. Bio-based phthalonitrile compounds: Synthesis, curing behavior, thermomechanical and thermal properties. React. Funct. Polym. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Han, J. Process design and techno-economic evaluation for catalytic production of cellulosic γ-Valerolactone using lignin derived propyl guaiacol. J. Ind. Eng. Chem. 2017, 52, 218–223. [Google Scholar] [CrossRef]
- García-Hidalgo, J.; Ravi, K.; Kuré, L.L.; Lidén, G.; Gorwa-Grauslund, M. Identification of the two-component guaiacol demethylase system from Rhodococcus rhodochrous and expression in Pseudomonas putida EM42 for guaiacol assimilation. AMB Express 2019, 9, 34. [Google Scholar] [CrossRef]
- Suitor, J.T.; Varzandeh, S.; Wallace, S. One-pot synthesis of adipic acid from guaiacol in Escherichia coli. ACS Synth. Biol. 2020, 9, 2472–2476. [Google Scholar] [CrossRef]
- van Duuren, J.; de Wild, P.J.; Starck, S.; Bradtmöller, C.; Selzer, M.; Mehlmann, K.; Schneider, R.; Kohlstedt, M.; Poblete-Castro, I.; Stolzenberger, J.; et al. Limited life cycle and cost assessment for the bioconversion of lignin-derived aromatics into adipic acid. Biotechnol. Bioeng. 2020, 117, 1381–1393. [Google Scholar] [CrossRef]
- Almqvist, H.; Veras, H.; Li, K.N.; Hidalgo, J.G.; Hulteberg, C.; Gorwa-Grauslund, M.; Parachin, N.S.; Carlquist, M. Muconic acid production using engineered Pseudomonas putida KT2440 and a guaiacol-rich fraction derived from kraft lignin. ACS Sustain. Chem. Eng. 2021, 9, 8097–8106. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Molina-Gutierrez, S.; Lacroix-Desmazes, P.; Caillol, S. Eugenol, a promising building block for biobased polymers with cutting-edge properties. Biomacromolecules 2021, 22, 3625–3648. [Google Scholar] [CrossRef]
- Moradipour, M.; Chase, E.K.; Khan, M.A.; Asare, S.O.; Lynn, B.C.; Rankin, S.E.; Knutson, B.L. Interaction of lignin-derived dimer and eugenol-functionalized silica nanoparticles with supported lipid bilayers. Colloid Surf. B-Biointerfaces 2020, 191, 111028. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.J.; Tarnavchyk, I.; Selvakumar, S.; Chisholm, B.J.; Sibi, M.; Webster, D.C. Poly (vinyl ethers) based on the biomass-derived compound, eugenol, and their one-component, ambient-cured surface coatings. Prog. Org. Coat. 2022, 170, 106996. [Google Scholar] [CrossRef]
- Li, X.F.; Lin, H.D.; Jiang, H.; Zhang, Y.Z.; Liu, B.H.; Sun, Y.A.; Zhao, C.J. Preparation and properties of a new bio-based epoxy resin/diatomite composite. Polym. Degrad. Stab. 2021, 187, 109541. [Google Scholar] [CrossRef]
- Singh, A.; Mukhopadhyay, K.; Sachan, S.G. Biotransformation of eugenol to vanillin by a novel strain Bacillus safensis SMS1003. Biocatal. Biotransform. 2019, 37, 291–303. [Google Scholar] [CrossRef]
- Lone, B.A.; Bhushan, A.; Ganjoo, A.; Katoch, M.; Gairola, S.; Gupta, P.; Babu, V. Biotransformation of eugenol by an endophytic fungus Daldinia sp. IIIMF4010 isolated from Rosmarinus officinalis. Nat. Prod. Res. 2022, 37, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ashengroph, M.; Amini, J. Bioconversion of isoeugenol to vanillin and vanillic acid using the resting cells of Trichosporon asahii. 3 Biotech 2017, 7, 358. [Google Scholar] [CrossRef]
- Lu, X.Y.; Wu, X.M.; Ma, B.D.; Xu, Y. Enhanced thermostability of Pseudomonas nitroreducens isoeugenol monooxygenase by the combinatorial strategy of surface residue replacement and consensus mutagenesis. Catalysts 2021, 11, 1199. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Xie, Y.M.; Chen, L.Y.; Xu, X.F.; Zha, C.X.; Cheng, F. Efficient biotransformation of isoeugenol to vanillin in recombinant strains of Escherichia coli by using engineered isoeugenol monooxygenase and sol-gel chitosan membrane. Proc. Biochem. 2018, 71, 76–81. [Google Scholar] [CrossRef]
- Sahu, P.; Ganesh, V.; Sakthivel, A. Oxidation of a lignin-derived-model compound: Iso-eugenol to vanillin over cerium containing MCM-22. Catal. Commun. 2020, 145, 106099. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Garcia, A.; Luque, R. Mechanochemical synthesis of graphene oxide-supported transition metal catalysts for the oxidation of isoeugenol to vanillin. Beilstein J. Org. Chem. 2017, 13, 1439–1445. [Google Scholar] [CrossRef]
- Franco, A.; de Souza, J.F.; do Nascimiento, P.F.P.; Pedroza, M.M.; de Carvalho, L.S.; Rodriguez-Castellón, E.; Luque, R. Sewage sludge-derived materials as efficient catalysts for the selective production of vanillin from isoeugenol. ACS Sustain. Chem. Eng. 2019, 7, 7519–7526. [Google Scholar] [CrossRef]
- Zuo, K.J.; Li, H.A.; Chen, J.H.; Ran, Q.P.; Huang, M.T.; Cui, X.X.; He, L.L.; Liu, J.S.; Jiang, Z.B. Effective biotransformation of variety of guaiacyl lignin monomers into vanillin by Bacillus pumilus. Front. Microbiol. 2022, 13, 901690. [Google Scholar] [CrossRef]
- Martínková, L.; Křístková, B.; Křen, V. Laccases and tyrosinases in organic synthesis. Int. J. Mol. Sci. 2022, 23, 3462. [Google Scholar] [CrossRef]
- Guazzaroni, M.; Pasqualini, M.; Botta, G.; Saladino, R. A novel synthesis of bioactive catechols by layer-by-layer immobilized tyrosinase in an organic solvent medium. ChemCatChem 2012, 4, 89–99. [Google Scholar] [CrossRef]
- Bozzini, T.; Botta, G.; Delfino, M.; Onofri, S.; Saladino, R.; Amatore, D.; Sgarbanti, R.; Nencioni, L.; Palamara, A.T. Tyrosinase and layer-by-layer supported tyrosinases in the synthesis of lipophilic catechols with antiinfluenza activity. Biorg. Med. Chem. 2013, 21, 7699–7708. [Google Scholar] [CrossRef]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Biorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Martini, A.; Serafini, F.; Aversa, D.; Piccinino, D.; Botta, L.; Berretta, N.; Guatteo, E.; Saladino, R. Tyrosinase mediated oxidative functionalization in the synthesis of DOPA-derived peptidomimetics with anti-Parkinson activity. RSC Adv. 2017, 7, 20502–20509. [Google Scholar] [CrossRef]
- Martínková, L.; Příhodová, R.; Kulik, N.; Pelantová, H.; Křístková, B.; Petrásková, L.; Biedermann, D. Biocatalyzed reactions towards functional food components 4-alkylcatechols and their analogues. Catalysts 2020, 10, 1077. [Google Scholar] [CrossRef]
- Senger, D.R.; Li, D.; Jaminet, S.C.; Cao, S.G. Activation of the Nrf2 cell defense pathway by ancient foods: Disease prevention by important molecules and microbes lost from the modern western diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef]
- Gygli, G.; Lucas, M.F.; Guallar, V.; van Berkel, W.J.H. The ins and outs of vanillyl alcohol oxidase: Identification of ligand migration paths. PLoS Comput. Biol. 2017, 13, e1005787. [Google Scholar] [CrossRef]
- Ewing, T.A.; Kühn, J.; Segarra, S.; Tortajada, M.; Zuhse, R.; van Berkel, W.J.H. Multigram scale enzymatic synthesis of (R)-1-(4′-Hydroxyphenyl)ethanol using vanillyl alcohol oxidase. Adv. Synth. Catal. 2018, 360, 2370–2376. [Google Scholar] [CrossRef]
- Gygli, G.; de Vries, R.P.; van Berkel, W.J.H. On the origin of vanillyl alcohol oxidases. Fungal Genet. Biol. 2018, 116, 24–32. [Google Scholar] [CrossRef] [PubMed]


























| Product | Substrate | Pros | Cons | Reference |
|---|---|---|---|---|
| Vanillin | Vanillic acid | Selectivity, high conversion, cofactor recycling | Purified enzyme costs | [44] |
| Ferulic acid | Selectivity | Purified enzyme and cofactor costs | [72] | |
| Selectivity, no cofactors | Purified enzyme costs | [73] | ||
| Isoeugenol | Selectivity, product purity, thermostable catalyst, low-cost substrate, isolated product (1 g) | Inhibition by vanillin 1 | [107] | |
| Vanillic acid | Ferulic acid | Low-cost substrate (rice bran), crystallized product (4 g) | Non-standard host | [78] |
| Syringaldehyde | Syringic acid | High conversion, no cofactors | Side product (alcohol) | [60] |
| Gallic acid | Vanillin | Convergent reactions, use of depolymerized lignin | Moderate concentrations of substrates | |
| Vanillic acid | ||||
| Syringic acid | [54] | |||
| p-Hydroxybenzoic acid | ||||
| Ferulic acid | ||||
| 4-Hydroxybenzoic acid | p-Coumaric acid | Selectivity, high conversion | Non-standard host; inhibition by substrate | [82] |
| cis,cis-Muconic acid | Guaiacol | High conversion; use of depolymerized lignin | Toxicity of catechol (intermediate) 2, side product (isomer) | [90,97,99] |
| Adipic acid | Catechol | High conversion | Moderate concentration of substrate | [97] |
| Guaiacol | Acceptable conversion | Guaiacol to catechol reaction rate-limiting | ||
| Methoxy- hydroquinone | Vanillin | Selectivity, acceptable conversion | Moderate concentration of substrate | [51] |
| Vanillic acid | ||||
| Alkylcatechols | Alkylphenols | Selectivity, high conversion, low-cost substrates, low-cost catalyst, products isolated | Moderate concentration of substrates | [114,118] |
| (R)-1-(4′- Hydroxy-phenyl)ethanol | 4-Ethylphenol | Stereoselectivity, low-cost substrate, product isolated (4 g) | Isolated yield moderate, O2 atmosphere required | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínková, L.; Grulich, M.; Pátek, M.; Křístková, B.; Winkler, M. Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules 2023, 13, 717. https://doi.org/10.3390/biom13050717
Martínková L, Grulich M, Pátek M, Křístková B, Winkler M. Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules. 2023; 13(5):717. https://doi.org/10.3390/biom13050717
Chicago/Turabian StyleMartínková, Ludmila, Michal Grulich, Miroslav Pátek, Barbora Křístková, and Margit Winkler. 2023. "Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review" Biomolecules 13, no. 5: 717. https://doi.org/10.3390/biom13050717
APA StyleMartínková, L., Grulich, M., Pátek, M., Křístková, B., & Winkler, M. (2023). Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules, 13(5), 717. https://doi.org/10.3390/biom13050717