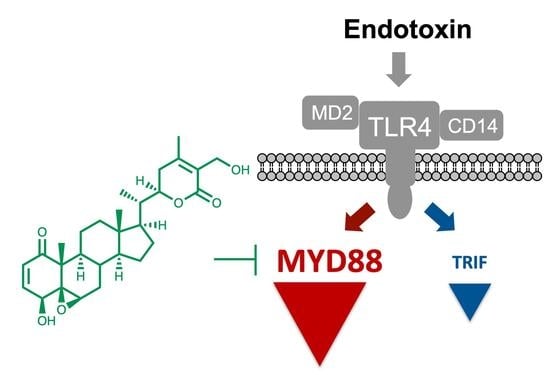
Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Treatment and Lysis for RT-qPCR Analysis
2.3. RT-qPCR Assay for the Detection of MyD88 and TRIF Pathways
2.4. In Vivo Cytokine Profiling
3. Results
3.1. Withaferin a Selectively Inhibits Pro-Inflammatory Signaling in DPL-Activated Macrophages
3.2. Withaferin a Selectively Attenuates Pro-Inflammatory Cytokine Responses in DPL-Treated Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A Comprehensive Review on Ethnopharmacology, Pharmacotherapeutics, Biomedicinal and Toxicological Aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Banerjee, S.; Biswas, S.; Das, B.; Kar, A.; Katiyar, C.K. Withania somnifera (L.) Dunal—Modern Perspectives of an Ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021, 264, 113157. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.; Roy, S.; Maity, R.; Mallick, A.; Soni, V.K.; Singh, P.K.; Chaurasiya, N.D.; Sangwan, R.S.; Misra-Bhattacharya, S.; Mandal, C. Chemotypical Variations in Withania somnifera Lead to Differentially Modulated Immune Response in BALB/c Mice. Vaccine 2012, 30, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Malik, F.; Suri, K.A.; Singh, J. Molecular Insight into the Immune Up-Regulatory Properties of the Leaf Extract of Ashwagandha and Identification of Th1 Immunostimulatory Chemical Entity. Vaccine 2009, 27, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Kuttan, G. Immunomodulatory Activity of Withania somnifera. J. Ethnopharmacol. 2000, 71, 193–200. [Google Scholar] [CrossRef]
- Ziauddin, M.; Phansalkar, N.; Patki, P.; Diwanay, S.; Patwardhan, B. Studies on the Immunomodulatory Effects of Ashwagandha. J. Ethnopharmacol. 1996, 50, 69–76. [Google Scholar] [CrossRef]
- Teixeira, S.T.; Valadares, M.C.; Gonçalves, S.A.; de Melo, A.; Queiroz, M.L.S. Prophylactic Administration of Withania somnifera Extract Increases Host Resistance in Listeria Monocytogenes Infected Mice. Int. Immunopharmacol. 2006, 6, 1535–1542. [Google Scholar] [CrossRef]
- Gautam, M.; Diwanay, S.S.; Gairola, S.; Shinde, Y.S.; Jadhav, S.S.; Patwardhan, B.K. Immune Response Modulation to DPT Vaccine by Aqueous Extract of Withania somnifera in Experimental System. Int. Immunopharmacol. 2004, 4, 841–849. [Google Scholar] [CrossRef]
- Saggam, A.; Limgaokar, K.; Borse, S.; Chavan-Gautam, P.; Dixit, S.; Tillu, G.; Patwardhan, B. Withania somnifera (L.) Dunal: Opportunity for Clinical Repurposing in COVID-19 Management. Front. Pharmacol. 2021, 12, 623795. [Google Scholar] [CrossRef]
- Eladl, A.H.; Mosad, S.M.; El-Shafei, R.A.; Saleh, R.M.; Ali, H.S.; Badawy, B.M.; Elshal, M.F. Immunostimulant Effect of a Mixed Herbal Extract on Infectious Bursal Disease Virus (IBDV) Vaccinated Chickens in the Context of a Co-Infection Model of Avian Influenza Virus H9N2 and IBDV. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101505. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Vazirian, M. Echinacea purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Saiki, I. Juzen-Taiho-To (Shi-Quan-Da-Bu-Tang): Scientific Evaluation and Clinical Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Hinz, B.; Woelkart, K.; Bauer, R. Alkamides from Echinacea Inhibit Cyclooxygenase-2 Activity in Human Neuroglioma Cells. Biochem. Biophys. Res. Commun. 2007, 360, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ban, J.; Xia, Y.; Zhou, F.; Yuan, C.; Jia, H.; Huang, H.; Jiang, M.; Liang, M.; Li, Z.; et al. Echinacea purpurea-Derived Homogeneous Polysaccharide Exerts Anti-Tumor Efficacy via Facilitating M1 Macrophage Polarization. Innovation 2023, 4, 100391. [Google Scholar] [CrossRef] [PubMed]
- Classen, B.; Thude, S.; Blaschek, W.; Wack, M.; Bodinet, C. Immunomodulatory Effects of Arabinogalactan-Proteins from Baptisia and Echinacea. Phytomedicine 2006, 13, 688–694. [Google Scholar] [CrossRef]
- Stimpel, M.; Proksch, A.; Wagner, H.; Lohmann-Matthes, M.L. Macrophage Activation and Induction of Macrophage Cytotoxicity by Purified Polysaccharide Fractions from the Plant Echinacea purpurea. Infect. Immun. 1984, 46, 845–849. [Google Scholar] [CrossRef]
- Luettig, B.; Steinmuller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.-L. Macrophage Activation by the Polysaccharide Arabinogalactan Isolated from Plant Cell Cultures of Echinacea purpurea. J. Natl. Cancer Inst. 1989, 81, 669–675. [Google Scholar] [CrossRef]
- Benson, J.M.; Pokorny, A.J.; Rhule, A.; Wenner, C.A.; Kandhi, V.; Cech, N.B.; Shepherd, D.M. Echinacea purpurea Extracts Modulate Murine Dendritic Cell Fate and Function. Food Chem. Toxicol. 2010, 48, 1170–1177. [Google Scholar] [CrossRef]
- Takaoka, A.; Iacovidou, M.; Hasson, T.; Montenegro, D.; Li, X.; Tsuji, M.; Kawamura, A. Biomarker-Guided Screening of Juzen-Taiho-to, an Oriental Herbal Formulation for Immunostimulation. Planta Med. 2014, 80, 283–289. [Google Scholar] [CrossRef]
- Todd, D.A.; Gulledge, T.V.; Britton, E.R.; Oberhofer, M.; Leyte-Lugo, M.; Moody, A.N.; Shymanovich, T.; Grubbs, L.F.; Juzumaite, M.; Graf, T.N.; et al. Ethanolic Echinacea purpurea Extracts Contain a Mixture of Cytokine-Suppressive and Cytokine-Inducing Compounds, Including Some That Originate from Endophytic Bacteria. PLoS ONE 2015, 10, e0124276. [Google Scholar] [CrossRef]
- Tamta, H.; Pugh, N.D.; Balachandran, P.; Moraes, R.; Sumiyanto, J.; Pasco, D.S. Variability in in Vitro Macrophage Activation by Commercially Diverse Bulk Echinacea Plant Material Is Predominantly Due to Bacterial Lipoproteins and Lipopolysaccharides. J. Agric. Food Chem. 2008, 56, 10552–10556. [Google Scholar] [CrossRef]
- Pugh, N.; Jackson, C.; Pasco, D. Total Bacterial Load within Echinacea purpurea, Determined Using a New PCR-Based Quantification Method, Is Correlated with LPS Levels and In Vitro Macrophage Activity. Planta Med. 2012, 79, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Haron, M.; Tyler, H.; Pugh, N.; Moraes, R.; Maddox, V.; Jackson, C.; Pasco, D. Activities and Prevalence of Proteobacteria Members Colonizing Echinacea purpurea Fully Account for Macrophage Activation Exhibited by Extracts of This Botanical. Planta Med. 2016, 82, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, D.; Kalpana, K.; Chrissian, C.; Sharma, A.; Takaoka, A.; Iacovidou, M.; Soll, C.E.; Aminova, O.; Heguy, A.; Cohen, L.; et al. Uncovering Potential ‘Herbal Probiotics’ in Juzen-Taiho-to through the Study of Associated Bacterial Populations. Bioorg. Med. Chem. Lett. 2015, 25, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, K.; Montenegro, D.; Romero, G.; Peralta, X.; Akgol Oksuz, B.; Heguy, A.; Tsuji, M.; Kawamura, A. Abundance of Plant-Associated Gammaproteobacteria Correlates with Immunostimulatory Activity of Angelica sinensis. Medicines 2019, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Issekutz, A.C. Removal of Gram-Negative Endotoxin from Solutions by Affinity Chromatography. J. Immunol. Methods 1983, 61, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, K.W.; Siebert, C.J. Efficient Endotoxin Removal with a New Sanitizable Affinity Column: Affi-Prep Polymyxin. J. Chromatogr. 1989, 476, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, K.; Yap, S.; Iyengar, R.; Tsuji, M.; Kawamura, A. Cell-line-based Assay for the Toxicity/Benefit Analysis of Lipopolysaccharides in Plants. Chem. Biol. Drug Des. 2020, 95, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Bazin-Lee, H.; Evans, J.T.; Casella, C.R.; Mitchell, T.C. MPL Adjuvant Contains Competitive Antagonists of Human TLR4. Front. Immunol. 2020, 11, 577823. [Google Scholar] [CrossRef] [PubMed]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.-B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Costa Clemens, S.A.; Dubin, G. Sustained Efficacy up to 4.5 Years of a Bivalent L1 Virus-like Particle Vaccine against Human Papillomavirus Types 16 and 18: Follow-up from a Randomised Control Trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef]
- Dubensky, T.W.J.; Reed, S.G. Adjuvants for Cancer Vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yarden, A.; Lavie, D. 567. Constituents of Withania somnifera. Part I. The Functional Groups of Withaferin. J. Chem. Soc. 1962, 2925–2927. [Google Scholar] [CrossRef]
- Logie, E.; Vanden Berghe, W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van der Veken, P.; Haegeman, G.; Vanden Berghe, W. Withaferin A Inhibits NF-KappaB Activation by Targeting Cysteine 179 in IKKβ. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef]
- Min, K.; Choi, K.; Kwon, T.K. Withaferin A Down-Regulates Lipopolysaccharide-Induced Cyclooxygenase-2 Expression and PGE2 Production through the Inhibition of STAT1/3 Activation in Microglial Cells. Int. Immunopharmacol. 2011, 11, 1137–1142. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, P.K.; Dudhe, R.; Singh, S. Biological Activities of Withania somnifera. Ann. Biol. Res. 2010, 1, 56–63. [Google Scholar]
- Meena, A.K.; Rekha, P.; Perumal, A.; Gokul, M.; Swathi, K.N.; Ilavarasan, R. Estimation of Withaferin-A by HPLC and Standardization of the Ashwagandhadi Lehyam Formulation. Heliyon 2021, 7, e06116. [Google Scholar] [CrossRef]
- Campbell, J.D. Development of the CpG Adjuvant 1018: A Case Study. In Vaccine Adjuvants; Fox, C.B., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1494, pp. 15–27. ISBN 978-1-4939-6443-7. [Google Scholar]
- Moser, B.A.; Escalante-Buendia, Y.; Steinhardt, R.C.; Rosenberger, M.G.; Cassaidy, B.J.; Naorem, N.; Chon, A.C.; Nguyen, M.H.; Tran, N.T.; Esser-Kahn, A.P. Small Molecule NF-ΚB Inhibitors as Immune Potentiators for Enhancement of Vaccine Adjuvants. Front. Immunol. 2020, 11, 511513. [Google Scholar] [CrossRef]
- Moser, B.A.; Steinhardt, R.C.; Escalante-Buendia, Y.; Boltz, D.A.; Barker, K.M.; Cassaidy, B.J.; Rosenberger, M.G.; Yoo, S.; McGonnigal, B.G.; Esser-Kahn, A.P. Increased Vaccine Tolerability and Protection via NF-ΚB Modulation. Sci. Adv. 2020, 6, eaaz8700. [Google Scholar] [CrossRef]
- Shen, H.; Tesar, B.M.; Walker, W.E.; Goldstein, D.R. Dual Signaling of MyD88 and TRIF Are Critical for Maximal TLR4- Induced Dendritic Cell Maturation. J. Immunol. 2008, 181, 1849–1858. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalpana, K.; Yap, S.; Tsuji, M.; Kawamura, A. Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera. Biomolecules 2023, 13, 828. https://doi.org/10.3390/biom13050828
Kalpana K, Yap S, Tsuji M, Kawamura A. Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera. Biomolecules. 2023; 13(5):828. https://doi.org/10.3390/biom13050828
Chicago/Turabian StyleKalpana, Kriti, Shen Yap, Moriya Tsuji, and Akira Kawamura. 2023. "Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera" Biomolecules 13, no. 5: 828. https://doi.org/10.3390/biom13050828
APA StyleKalpana, K., Yap, S., Tsuji, M., & Kawamura, A. (2023). Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera. Biomolecules, 13(5), 828. https://doi.org/10.3390/biom13050828