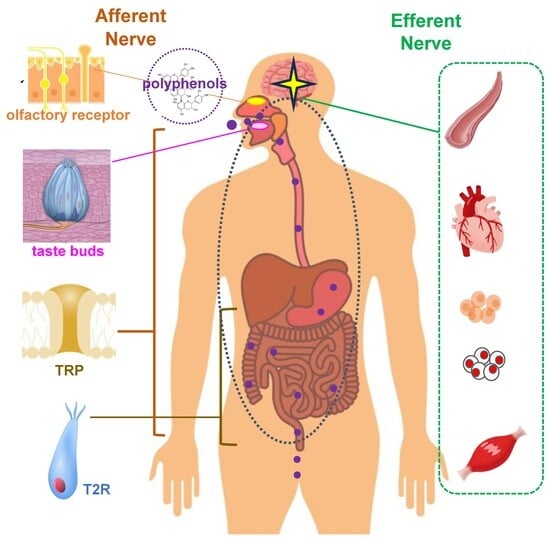
Sensory Nutrition and Bitterness and Astringency of Polyphenols
Abstract
:1. Introduction
2. Receptors Involved in Sensory Nutrition
3. Bitter Taste Receptors and Polyphenols
3.1. Extra-Oral Bitter Taste Receptor and Gastrointestinal Hormones
3.2. Interactions between Bitter Taste Receptors and Polyphenols
4. Astringent Sensor and Polyphenols
4.1. Mechanisms of Recognition for Astringency Perception
4.2. Astringency Perception Is a Stressor
4.3. Astringent Polyphenols and TRP Channels
5. Bioavailability of Polyphenols
6. Biological Regulation through Bitterness and Astringency of Polyphenols
6.1. Circulation and Polyphenol
6.2. Blood Glucose and Polyphenols
6.3. Obesity and Polyphenols
6.4. Brain Function and Polyphenol
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 317s–325s. [Google Scholar] [CrossRef]
- Dabas, D. Polyphenols as Colorants. Adv. Food Technol. Nutr. Sci. Open J. 2018, SE, S1–S6. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 665–1533. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Quijada-Morín, N.; Brás, N.F.; Gomes, P.; de Freitas, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterization of Sensory Properties of Flavanols—A Molecular Dynamic Approach. Chem. Senses 2015, 40, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Labbe, D.; Damevin, L.; Vaccher, C.; Morgenegg, C.; Martin, N. Modulation of perceived taste by olfaction in familiar and unfamiliar beverages. Food Qual. Prefer. 2006, 17, 582–589. [Google Scholar] [CrossRef]
- Mattes, R.D. Influences on acceptance of bitter foods and beverages. Physiol. Behav. 1994, 56, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.J.; Nagai, H.; Nakagawa, M.; Beauchamp, G.K. Effects of repeated exposure and health-related information on hedonic evaluation and acceptance of a bitter beverage. Appetite 2003, 40, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Tripodo, G.; Santi, L.; Fanali, C. Cocoa Polyphenols: Chemistry, Bioavailability and Effects on Cardiovascular Performance. Curr. Med. Chem. 2018, 25, 4903–4917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Molsberry, S.A.; Yeh, T.S.; Cassidy, A.; Schwarzschild, M.A.; Ascherio, A.; Gao, X. Intake of Flavonoids and Flavonoid-Rich Foods and Mortality Risk Among Individuals with Parkinson Disease: A Prospective Cohort Study. Neurology 2022, 98, e1064–e1076. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Tang, D.; Tran, Y.; Shekhawat, G.S.; Gopinath, B. Dietary Flavonoid Intake and Chronic Sensory Conditions: A Scoping Review. Antioxidants 2022, 11, 1214. [Google Scholar] [CrossRef]
- Imamura, F.; Schulze, M.B.; Sharp, S.J.; Guevara, M.; Romaguera, D.; Bendinelli, B.; Salamanca-Fernández, E.; Ardanaz, E.; Arriola, L.; Aune, D.; et al. Estimated Substitution of Tea or Coffee for Sugar-Sweetened Beverages Was Associated with Lower Type 2 Diabetes Incidence in Case-Cohort Analysis across 8 European Countries in the EPIC-InterAct Study. J. Nutr. 2019, 149, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, Y.; Alperet, D.J.; Liu, G.; Malik, V.; Manson, J.E.; Rimm, E.B.; Hu, F.B.; Sun, Q. Beverage consumption and mortality among adults with type 2 diabetes: Prospective cohort study. BMJ 2023, 381, e073406. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V.; et al. Effect of cocoa flavanol supplementation for prevention of cardiovascular disease events: The COSMOS randomized clinical trial. Am. J. Clin. Nutr. 2022, 115, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Yeung, L.K.; Alschuler, D.M.; Ottaviani, J.I.; Kuhnle, G.G.C.; Sloan, R.P.; Luttmann-Gibson, H.; Copeland, T.; Schroeter, H.; Sesso, H.D.; et al. Dietary flavanols restore hippocampal-dependent memory in older adults with lower diet quality and lower habitual flavanol consumption. Proc. Natl. Acad. Sci. USA 2023, 120, e2216932120. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Terao, J. Possible mechanisms of postprandial physiological alterations following flavan 3-ol ingestion. Nutr. Rev. 2018, 76, 174–186. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Mainland, J.D.; Arayata, C.J. Sensory nutrition: The role of taste in the reviews of commercial food products. Physiol. Behav. 2019, 209, 112579. [Google Scholar] [CrossRef]
- Reed, D.R.; Alhadeff, A.L.; Beauchamp, G.K.; Chaudhari, N.; Duffy, V.B.; Dus, M.; Fontanini, A.; Glendinning, J.I.; Green, B.G.; Joseph, P.V.; et al. NIH Workshop Report: Sensory nutrition and disease. Am. J. Clin. Nutr. 2021, 113, 232–245. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Depoortere, I.; Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 2019, 18, 116–138. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef]
- Kato, A.; Touhara, K. Mammalian olfactory receptors: Pharmacology, G protein coupling and desensitization. Cell Mol. Life Sci. 2009, 66, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Munger, S.D. Olfactory receptors: G protein-coupled receptors and beyond. J. Neurochem. 2009, 109, 1570–1583. [Google Scholar] [CrossRef]
- Roper, S.D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014, 223, 827–871. [Google Scholar] [PubMed]
- Zufall, F. TRPs in olfaction. Handb. Exp. Pharmacol. 2014, 223, 917–933. [Google Scholar]
- Green, B.G. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem. Senses 2012, 37, 201–206. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Jang, Y. Inhaled Volatile Molecules-Responsive TRP Channels as Non-Olfactory Receptors. Biomol. Ther. 2023. [Google Scholar] [CrossRef]
- Guichard, E.; Barba, C.; Thomas-Danguin, T.; Tromelin, A. Multivariate Statistical Analysis and Odor-Taste Network to Reveal Odor-Taste Associations. J. Agric. Food Chem. 2020, 68, 10318–10328. [Google Scholar] [CrossRef]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Tong, T.; Wang, Y.; Kang, S.G.; Huang, K. Ectopic Odorant Receptor Responding to Flavor Compounds: Versatile Roles in Health and Disease. Pharmaceutics 2021, 13, 1314. [Google Scholar] [CrossRef]
- D’Urso, O.; Drago, F. Pharmacological significance of extra-oral taste receptors. Eur. J. Pharmacol. 2021, 910, 174480. [Google Scholar] [CrossRef]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J.; et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Park, J.; Moon, C.; Park, T. Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells. Nutrients 2018, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Giusepponi, M.E.; Kern, M.; Chakaroun, R.; Wohland, T.; Kovacs, P.; Dietrich, A.; Schön, M.R.; Krohn, K.; Pucci, M.; Polidori, C.; et al. Gene expression profiling in adipose tissue of Sprague Dawley rats identifies olfactory receptor 984 as a potential obesity treatment target. Biochem. Biophys. Res. Commun. 2018, 505, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 379–385. [Google Scholar] [CrossRef]
- Zhai, K.; Yang, Z.; Zhu, X.; Nyirimigabo, E.; Mi, Y.; Wang, Y.; Liu, Q.; Man, L.; Wu, S.; Jin, J.; et al. Activation of bitter taste receptors (tas2rs) relaxes detrusor smooth muscle and suppresses overactive bladder symptoms. Oncotarget 2016, 7, 21156–21167. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef]
- Ansoleaga, B.; Garcia-Esparcia, P.; Pinacho, R.; Haro, J.M.; Ramos, B.; Ferrer, I. Decrease in olfactory and taste receptor expression in the dorsolateral prefrontal cortex in chronic schizophrenia. J. Psychiatr. Res. 2015, 60, 109–116. [Google Scholar] [CrossRef]
- Foster, S.R.; Blank, K.; See Hoe, L.E.; Behrens, M.; Meyerhof, W.; Peart, J.N.; Thomas, W.G. Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 4497–4508. [Google Scholar] [CrossRef]
- Tizzano, M.; Finger, T.E. Chemosensors in the nose: Guardians of the airways. Physiology 2013, 28, 51–60. [Google Scholar] [CrossRef]
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013, 19, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.C.; Kobs, A.J.; Kramer, A.; Nyquist, M.; Kuroki, M.T.; Osborn, J.; Lidke, D.S.; Low-Nam, S.T.; Blazar, B.R.; Tolar, J. Bone Marrow Stromal and Vascular Smooth Muscle Cells Have Chemosensory Capacity via Bitter Taste Receptor Expression. PLoS ONE 2013, 8, e58945. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Vancleef, L.; Van Den Broeck, T.; Thijs, T.; Steensels, S.; Briand, L.; Tack, J.; Depoortere, I. Chemosensory signalling pathways involved in sensing of amino acids by the ghrelin cell. Sci. Rep. 2015, 5, 15725. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Taste perception: From the tongue to the testis. Mol. Hum. Reprod. 2013, 19, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Sowers, J.R. Mineralocorticoid antagonists and ENaC inhibitors in hyperaldosteronism. J. Clin. Hypertens. 2019, 21, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.P.; Ahuja, E.; Lin, H.; Qi, J.; He, Q.; Gao, S.; An, H.; Zhang, J.; Xie, Y.; Liang, D. A Review on the Role of TRP Channels and Their Potential as Drug Targets_An Insight Into the TRP Channel Drug Discovery Methodologies. Front. Pharmacol. 2022, 13, 914499. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Diakogiannaki, E.; Gentry, C.; Psichas, A.; Habib, A.M.; Bevan, S.; Fischer, M.J.; Reimann, F.; Gribble, F.M. Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes 2015, 64, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Gunawan, A.L.; Tso, P.; Aponte, G.W. Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide stimulate release of substance P from TRPV1- and TRPA1-expressing sensory nerves. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G23–G35. [Google Scholar] [CrossRef]
- Yan, J.; Tong, H. An overview of bitter compounds in foodstuffs: Classifications, evaluation methods for sensory contribution, separation and identification techniques, and mechanism of bitter taste transduction. Compr. Rev. Food Sci. Food Saf. 2023, 22, 187–232. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Niv, M.Y.; Meyerhof, W. Structural requirements of bitter taste receptor activation. Proc. Natl. Acad. Sci. USA 2010, 107, 11110–11115. [Google Scholar] [CrossRef]
- Meyerhof, W. Elucidation of mammalian bitter taste. Rev. Physiol. Biochem. Pharmacol. 2005, 154, 37–72. [Google Scholar]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef]
- Lossow, K.; Hübner, S.; Roudnitzky, N.; Slack, J.P.; Pollastro, F.; Behrens, M.; Meyerhof, W. Comprehensive Analysis of Mouse Bitter Taste Receptors Reveals Different Molecular Receptive Ranges for Orthologous Receptors in Mice and Humans. J. Biol. Chem. 2016, 291, 15358–15377. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors: Genes, evolution and health. Evol. Med. Public. Health 2021, 9, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liddle, R.A. Gastrointestinal hormones and the gut connectome. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4827–48231. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Harikumar, K.G.; Wootten, D.; Sexton, P.M. Roles of Cholecystokinin in the Nutritional Continuum. Physiology and Potential Therapeutics. Front. Endocrinol. 2021, 12, 684656. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.H.; Balsiger, L.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The gastrointestinal tract in hunger and satiety signalling. United Eur. Gastroenterol. J. 2021, 9, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef]
- Zhao, T.C. Glucagon-like peptide-1 (GLP-1) and protective effects in cardiovascular disease: A new therapeutic approach for myocardial protection. Cardiovasc. Diabetol. 2013, 12, 90. [Google Scholar] [CrossRef]
- Christensen, M.; Bagger, J.I.; Vilsbøll, T.; Knop, F.K. The alpha-cell as target for type 2 diabetes therapy. Rev. Diabet. Stud. 2011, 8, 369–381. [Google Scholar] [CrossRef]
- Deacon, C.F.; Ahrén, B. Physiology of incretins in health and disease. Rev. Diabet. Stud. 2011, 8, 293–306. [Google Scholar] [CrossRef]
- Rezaie, P.; Bitarafan, V.; Rose, B.D.; Lange, K.; Mohammadpour, Z.; Rehfeld, J.F.; Horowitz, M.; Feinle-Bisset, C. Effects of Quinine on the Glycaemic Response to, and Gastric Emptying of, a Mixed-Nutrient Drink in Females and Males. Nutrients 2023, 15, 3584. [Google Scholar] [CrossRef]
- Rose, B.D.; Bitarafan, V.; Rezaie, P.; Fitzgerald, P.C.E.; Horowitz, M.; Feinle-Bisset, C. Comparative Effects of Intragastric and Intraduodenal Administration of Quinine on the Plasma Glucose Response to a Mixed-Nutrient Drink in Healthy Men: Relations with Glucoregulatory Hormones and Gastric Emptying. J. Nutr. 2021, 151, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Verbeure, W.; Deloose, E.; Tóth, J.; Rehfeld, J.F.; Van Oudenhove, L.; Depoortere, I.; Tack, J. The endocrine effects of bitter tastant administration in the gastrointestinal system: Intragastric versus intraduodenal administration. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Rowling, M.J.; Pritchard, S.K. Possible role of type 1 and type 2 taste receptors on obesity-induced inflammation. Nutr. Rev. 2022, 80, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Medapati, M.R.; Bhagirath, A.Y.; Singh, N.; Chelikani, P. Pharmacology of T2R Mediated Host-Microbe Interactions. Handb. Exp. Pharmacol. 2022, 275, 177–202. [Google Scholar] [PubMed]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Tarragon, E.; Moreno, J.J. Polyphenols and taste 2 receptors. Physiological, pathophysiological and pharmacological implications. Biochem. Pharmacol. 2020, 178, 114086. [Google Scholar] [CrossRef]
- Behrens, M.; Brockhoff, A.; Batram, C.; Kuhn, C.; Appendino, G.; Meyerhof, W. The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. J. Agric. Food Chem. 2009, 57, 9860–9866. [Google Scholar] [CrossRef]
- Roland, W.S.; Vincken, J.P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar] [CrossRef]
- Yamazaki, T.; Narukawa, M.; Mochizuki, M.; Misaka, T.; Watanabe, T. Activation of the hTAS2R14 human bitter-taste receptor by (−)-epigallocatechin gallate and (−)-epicatechin gallate. Biosci. Biotechnol. Biochem. 2013, 77, 1981–1983. [Google Scholar] [CrossRef]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chemosens. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Roland, W.S.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.P. Bitter taste receptor activation by flavonoids and isoflavonoids: Modeled structural requirements for activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Misaka, T.; Ishiguro, M.; Masuda, K.; Sugawara, T.; Ito, K.; Kobayashi, T.; Matsuo, S.; Ishimaru, Y.; Asakura, T.; et al. Characterization of the beta-D-glucopyranoside binding site of the human bitter taste receptor hTAS2R16. J. Biol. Chem. 2010, 285, 28373–28378. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Ikeda, R.; Yamazaki, T.; Ito, K.; Uda, K.; Wakabayashi, K.; Watanabe, T. Activation of human bitter taste receptors by polymethoxylated flavonoids. Biosci. Biotechnol. Biochem. 2016, 80, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 223s–229s. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.R.; Sadiq, N.M. Neuroanatomy, Neural Taste Pathway. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef] [PubMed]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Gastrophysics of the Oral Cavity. Curr. Pharm. Des. 2016, 22, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Sadachi, H.; Nakamura, J.; Tonoike, M. Functional magnetic resonance imaging investigation of brain regions associated with astringency. Neurosci. Res. 2017, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano-Bailón, M.T. Interactions between wine phenolic compounds and human saliva in astringency perception. Food Funct. 2018, 9, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rochera, B.; Manjón, E.; Escribano-Bailón, M.T.; García-Estévez, I. Role of Anthocyanins in the Interaction between Salivary Mucins and Wine Astringent Compounds. Foods 2023, 12, 3623. [Google Scholar] [CrossRef]
- Takahashi, S.; Kurogi, M.; Saitoh, O. The diversity in sensitivity of TRPA1 and TRPV1 of various animals to polyphenols. Biomed. Res. 2021, 42, 43–51. [Google Scholar] [CrossRef]
- Kurogi, M.; Miyashita, M.; Emoto, Y.; Kubo, Y.; Saitoh, O. Green tea polyphenol epigallocatechin gallate activates TRPA1 in an intestinal enteroendocrine cell line, STC-1. Chem. Senses 2012, 37, 167–177. [Google Scholar] [CrossRef]
- Kurogi, M.; Kawai, Y.; Nagatomo, K.; Tateyama, M.; Kubo, Y.; Saitoh, O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem. Senses 2015, 40, 27–46. [Google Scholar] [CrossRef]
- Amoah, I.; Lim, J.J.; Osei, E.O.; Arthur, M.; Tawiah, P.; Oduro, I.N.; Aduama-Larbi, M.S.; Lowor, S.T.; Rush, E. Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis. Foods 2022, 11, 1962. [Google Scholar] [CrossRef]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.R.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2012, Cd008893. [Google Scholar] [CrossRef]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Ebaditabar, M.; Djafarian, K.; Saeidifard, N.; Shab-Bidar, S. Effect of dark chocolate on flow-mediated dilatation: Systematic review, meta-analysis, and dose-response analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 36, 17–27. [Google Scholar] [CrossRef]
- Osakabe, N.; Fushimi, T.; Fujii, Y. Hormetic response to B-type procyanidin ingestion involves stress-related neuromodulation via the gut-brain axis: Preclinical and clinical observations. Front. Nutr. 2022, 9, 969823. [Google Scholar] [CrossRef]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-response relationship between cocoa flavanols and human endothelial function: A systematic review and meta-analysis of randomized trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Osakabe, N.; Di Paola, R.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Fritsch, T.; Abdelhameed, A.S.; et al. Hormesis defines the limits of lifespan. Ageing Res. Rev. 2023, 91, 102074. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Asp. Med. 2011, 32, 279–304. [Google Scholar] [CrossRef]
- Cornelius, C.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Hormesis and vitagenes in aging and longevity: Mitochondrial control and hormonal regulation. Horm. Mol. Biol. Clin. Investig. 2013, 16, 73–89. [Google Scholar] [CrossRef]
- Fushimi, T.; Fujii, Y.; Koshino, H.; Inagawa, K.; Saito, A.; Koizumi, R.; Shibata, M.; Osakabe, N. Method for detecting hemodynamic alterations following a single gavage in rats. Exp. Anim. 2021, 70, 372–377. [Google Scholar] [CrossRef]
- Ingawa, K.; Aruga, N.; Matsumura, Y.; Shibata, M.; Osakabe, N. Alteration of the systemic and microcirculation by a single oral dose of flavan-3-ols. PLoS ONE 2014, 9, e94853. [Google Scholar] [CrossRef]
- Saito, A.; Inagawa, K.; Ebe, R.; Fukase, S.; Horikoshi, Y.; Shibata, M.; Osakabe, N. Onset of a hypotensive effect following ingestion of flavan 3-ols involved in the activation of adrenergic receptors. Free Radic. Biol. Med. 2016, 99, 584–592. [Google Scholar] [CrossRef]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin. Proc. 2009, 84, 822–830. [Google Scholar] [CrossRef]
- Charkoudian, N.; Wallin, B.G. Sympathetic neural activity to the cardiovascular system: Integrator of systemic physiology and interindividual characteristics. Compr. Physiol. 2014, 4, 825–850. [Google Scholar] [PubMed]
- Koizumi, R.; Fushimi, T.; Sato, Y.; Fujii, Y.; Sato, H.; Osakabe, N. Relationship between hemodynamic alteration and sympathetic nerve activation following a single oral dose of cinnamtannin A2. Free Radic. Res. 2021, 55, 491–498. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Horikoshi, Y.; Fushimi, T.; Saito, A.; Koizumi, R.; Fujii, Y.; Hu, Q.Q.; Hirota, Y.; Aizawa, K.; Osakabe, N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct. 2019, 10, 1726–1735. [Google Scholar] [CrossRef]
- Tentolouris, N.; Liatis, S.; Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 129–152. [Google Scholar] [CrossRef]
- Ishii, Y.; Muta, O.; Teshima, T.; Hirasima, N.; Odaka, M.; Fushimi, T.; Fujii, Y.; Osakabe, N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients 2021, 13, 4214. [Google Scholar] [CrossRef]
- Muta, O.; Oyama, S.; Odaka, M.; Shimizu, K.; Katsuragawa, S.; Suzuki, K.; Fushimi, T.; Fujii, Y.; Akagi, R.; Osakabe, N. Cinnamtannin A2, (−)-epicatechin tetramer, attenuates skeletal muscle wasting in disuse atrophy model mice induced by hindlimb suspension. J. Clin. Biochem. Nutr. 2023, 73, 124–130. [Google Scholar] [CrossRef]
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharmacol. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ishimura, K.; Oya, S.; Kamino, M.; Fujii, Y.; Nanba, F.; Toda, T.; Ishii, T.; Adachi, T.; Suhara, Y.; et al. Comparison of the sympathetic stimulatory abilities of B-type procyanidins based on induction of uncoupling protein-1 in brown adipose tissue (BAT) and increased plasma catecholamine (CA) in mice. PLoS ONE 2018, 13, e0201203. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Kurokawa, T.; Mori, Y. Sensing of redox status by TRP channels. Cell Calcium 2016, 60, 115–122. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Trovato, A.; Cavallaro, M.; Mancuso, C.; Di Rienzo, L.; Condorelli, D.; De Lorenzo, A.; Calabrese, E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010, 16, 877–883. [Google Scholar] [CrossRef]
- Molina-Hidalgo, C.; Stillman, C.M.; Collins, A.M.; Velazquez-Diaz, D.; Ripperger, H.S.; Drake, J.A.; Gianaros, P.J.; Marsland, A.L.; Erickson, K.I. Changes in stress pathways as a possible mechanism of aerobic exercise training on brain health: A scoping review of existing studies. Front. Physiol. 2023, 14, 1273981. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Broderick, A.V.; Loughlin-Presnal, J.E.; Bendezu, J.J.; Joos, C.M.; Ahlkvist, J.A.; Perzow, S.E.D.; McDonald, A. Co-activation of SAM and HPA responses to acute stress: A review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Dev. Psychobiol. 2019, 61, 1079–1093. [Google Scholar] [CrossRef]
- Fujii, Y.; Suzuki, K.; Adachi, T.; Taira, S.; Osakabe, N. Corticotropin-releasing hormone is significantly upregulated in the mouse paraventricular nucleus following a single oral dose of cinnamtannin A2 as an (−)-epicatechin tetramer. J. Clin. Biochem. Nutr. 2019, 65, 29–33. [Google Scholar] [CrossRef]
- Fujii, Y.; Suzuki, K.; Hasegawa, Y.; Nanba, F.; Toda, T.; Adachi, T.; Taira, S.; Osakabe, N. Single oral administration of flavan 3-ols induces stress responses monitored with stress hormone elevations in the plasma and paraventricular nucleus. Neurosci. Lett. 2018, 682, 106–111. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef]
- Yu, X.; Yu, M.; Liu, Y.; Yu, S. TRP channel functions in the gastrointestinal tract. Semin. Immunopathol. 2016, 38, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Dezaki, K.; Yoneshiro, T.; Watanabe, T.; Yamazaki, J.; Saito, M.; Yada, T.; Tominaga, M.; Iwasaki, Y. Involvement of thermosensitive TRP channels in energy metabolism. J. Physiol. Sci. 2017, 67, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.H. Neural control of blood pressure: Focusing on capsaicin-sensitive sensory nerves. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, T.; Hirahata, C.; Hiroki, K.; Fujii, Y.; Calabrese, V.; Suhara, Y.; Osakabe, N. Activation of transient receptor potential channels is involved in reactive oxygen species (ROS)-dependent regulation of blood flow by (−)-epicatechin tetramer cinnamtannin A2. Biochem. Pharmacol. 2023, 214, 115682. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Duke, S.O. Function of polyphenol oxidase in higher plants. Physiol. Plant. 1984, 60, 106–112. [Google Scholar] [CrossRef]
- Tan, J.; de Bruijn, W.J.C.; van Zadelhoff, A.; Lin, Z.; Vincken, J.-P. Browning of Epicatechin (EC) and Epigallocatechin (EGC) by Auto-Oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal. 2014, 21, 971–986. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Fujiwara, K.; Polat, O.K.; Badr, H.; Takahashi, N.; Mori, Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification. J. Biol. Chem. 2016, 291, 4197–4210. [Google Scholar] [CrossRef]
- Kawase, M.; Chen, W.; Kawaguchi, K.; Nyasha, M.R.; Sasaki, S.; Hatakeyama, H.; Kaneko, T.; Kanzaki, M. TRPA1 and TRPV1 channels participate in atmospheric-pressure plasma-induced [Ca2+]i response. Sci. Rep. 2020, 10, 9687. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: Insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 230s–242s. [Google Scholar] [CrossRef]
- Felgines, C.; Talavéra, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Sonntag, S.; Strass, G.; Frank, T.; Bitsch, I. Urinary Excretion of Cyanidin Glucosides and Glucuronides in Healthy Humans After Elderberry Juice Ingestion. J. Biomed. Biotechnol. 2004, 2004, 343–345. [Google Scholar] [CrossRef]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym. Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between Gut Microbiota and Polyphenols: New Insights into the Treatment of Fatigue. Molecules 2022, 27, 7377. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Zhang, X. Dietary Polyphenols as Prospective Natural-Compound Depression Treatment from the Perspective of Intestinal Microbiota Regulation. Molecules 2022, 27, 7637. [Google Scholar] [CrossRef]
- Rojas, M.; Chávez-Castillo, M.; Pirela, D.; Parra, H.; Nava, M.; Chacín, M.; Angarita, L.; Añez, R.; Salazar, J.; Ortiz, R.; et al. Metabolic Syndrome: Is It Time to Add the Central Nervous System? Nutrients 2021, 13, 2254. [Google Scholar] [CrossRef]
- Kiyimba, T.; Yiga, P.; Bamuwamye, M.; Ogwok, P.; Van der Schueren, B.; Matthys, C. Efficacy of Dietary Polyphenols from Whole Foods and Purified Food Polyphenol Extracts in Optimizing Cardiometabolic Health: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 270–282. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipoor, N.; Shafiee, F.; Rostami, A.; Kahrizi, M.S.; Soleimanpour, H.; Ghodsi, M.; Ansari, M.J.; Bokov, D.O.; Jannat, B.; Mosharkesh, E.; et al. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2022, 36, 3529–3539. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, X.X.; Bai, Y.Y.; Wang, X.J.; Sun, K.; Chen, J.Z.; Hui, R.T. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: A meta-analysis of randomized placebo-controlled trials. Am. J. Clin. Nutr. 2010, 91, 480–486. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef]
- Watanabe, N.; Inagawa, K.; Shibata, M.; Osakabe, N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis. 2014, 13, 64. [Google Scholar] [CrossRef]
- Fushimi, T.; Oyama, S.; Koizumi, R.; Fujii, Y.; Osakabe, N. Impact of cyanidin 3-O-glucoside on rat micro-and systemic circulation, possibly thorough angiogenesis. J. Clin. Biochem. Nutr. 2023, 72, 132–138. [Google Scholar] [CrossRef]
- Resnick, N.; Yahav, H.; Shay-Salit, A.; Shushy, M.; Schubert, S.; Zilberman, L.C.; Wofovitz, E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog. Biophys. Mol. Biol. 2003, 81, 177–199. [Google Scholar] [CrossRef]
- Li, Y.S.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Gorski, T.; De Bock, K. Metabolic regulation of exercise-induced angiogenesis. Vasc. Biol. 2019, 1, H1–H8. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Galie, P.A.; Nguyen, D.H.; Choi, C.K.; Cohen, D.M.; Janmey, P.A.; Chen, C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2014, 111, 7968–7973. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Saito, S.; Hanada, R.; Hibi, M. Acute Dose-Response Effectiveness of Combined Catechins and Chlorogenic Acids on Postprandial Glycemic Responses in Healthy Men: Results from Two Randomized Studies. Nutrients 2023, 15, 777. [Google Scholar] [CrossRef]
- Reis, C.E.G.; Paiva, C.; Amato, A.A.; Lofrano-Porto, A.; Wassell, S.; Bluck, L.J.C.; Dórea, J.G.; da Costa, T.H.M. Decaffeinated coffee improves insulin sensitivity in healthy men. Br. J. Nutr. 2018, 119, 1029–1038. [Google Scholar] [CrossRef]
- Jokura, H.; Watanabe, I.; Umeda, M.; Hase, T.; Shimotoyodome, A. Coffee polyphenol consumption improves postprandial hyperglycemia associated with impaired vascular endothelial function in healthy male adults. Nutr. Res. 2015, 35, 873–881. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.J.; Huang, L.H.; Chen, I.J.; Chiu, J.P.; Hsu, C.H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: A randomized, double-blinded, and placebo-controlled trial. PLoS ONE 2014, 9, e91163. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Matsumae, T.; Kataoka, T.; Yazaki, Y.; Yamaguchi, H. Effect of acacia polyphenol on glucose homeostasis in subjects with impaired glucose tolerance: A randomized multicenter feeding trial. Exp. Ther. Med. 2013, 5, 1566–1572. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Balasooriya, H.; Sirisena, S.; Ng, K. The effectiveness of dietary polyphenols in obesity management: A systematic review and meta-analysis of human clinical trials. Food Chem. 2023, 404 Pt B, 134668. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Xiang, J.Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Raven, L.M.; Stoita, A.; Feller, R.B.; Brown, C.; Greenfield, J.R. Delayed Gastric Emptying with Perioperative Use of Glucagon-like Peptide-1 Receptor Agonists. Am. J. Med. 2023, 136, e233–e234. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef]
- Fraser, K.A.; Davison, J.S. Cholecystokinin-induced c-fos expression in the rat brain stem is influenced by vagal nerve integrity. Exp. Physiol. 1992, 77, 225–228. [Google Scholar] [CrossRef]
- Rinaman, L.; Baker, E.A.; Hoffman, G.E.; Stricker, E.M.; Verbalis, J.G. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am. J. Physiol. 1998, 275, R262–R268. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Hsu, T.M.; Liu, C.M.; Noble, E.E.; Cortella, A.M.; Nakamoto, E.M.; Hahn, J.D.; de Lartigue, G.; Kanoski, S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 2018, 9, 2181. [Google Scholar] [CrossRef] [PubMed]
- Klarer, M.; Arnold, M.; Günther, L.; Winter, C.; Langhans, W.; Meyer, U. Gut vagal afferents differentially modulate innate anxiety and learned fear. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 7067–7076. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Yan, Q.; Ma, Y.; Fang, J.; Yang, Y. Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front. Neurol. 2022, 13, 1015175. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, J.L.; Valldecabres, R.; Villarrasa-Sapiña, I.; Monfort-Torres, G.; Marco-Ahulló, A.; Ribeiro Do Couto, B. Increased cortisol levels caused by acute resistance physical exercise impair memory and learning ability. PeerJ 2022, 10, e13000. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and growth hormone responses to exercise at different times of day. J. Clin. Endocrinol. Metab. 2001, 86, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med. 2008, 10, 118–127. [Google Scholar] [CrossRef]
- Adlard, P.A.; Cotman, C.W. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 2004, 124, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012, 233, 12–21. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; Kitaichi, Y.; An, Y.; Omiya, Y.; Song, N.; Koga, M.; Kato, A.; Inoue, T.; Kusumi, I. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 2016, 69, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Fujii, Y.; Sakata, J.; Sato, F.; Onishi, K.; Yamato, Y.; Sakata, K.; Taira, S.; Sato, H.; Osakabe, N. Impact of short-term oral dose of cinnamtannin A2, an (−)-epicatechin tetramer, on spatial memory and adult hippocampal neurogenesis in mouse. Biochem. Biophys. Res. Commun. 2021, 585, 1–7. [Google Scholar] [CrossRef]




| T2R | Compounds |
|---|---|
| 1 | Isoxanthohumol (85) Resveratrol (60) Xanthohumol (85) Amarogentin (87) |
| 3 | ND |
| 4 | (−)-Epicatechin (3) Epigallocatechin gallate (4) Amarogentin (87) |
| 5 | (−)-Epicatechin (3) Epigallocatechin gallate (4) Procyanidin B1 (4) Procyanidin B2g (4) Procyanidin B4 (4) Procyanidin B7 (4) Procyanidin C2 (3) PGG(pentagalloylglucose) (3) Punicalagin (4) |
| 7 | Procyanidin B1 (4) Malvidin-3-glucoside (3) Castalagin (4) Grandinin (4) Punicalagin (4) Vescalagin (4) |
| 8 | ND |
| 9 | ND |
| 10 | Amarogentin (87) |
| 13 | ND |
| 60 | (+)-Catechin (86) (−)-Epicatechin gallate (84) Epigallocatechin gallate (84) Eriodictyol (86) Flavanone (86) Hesperitin (86) Homoeriodictyol (86) Liquiritigenin (86) Naringenin (86) 8-Prenylnaringenin (85) Pinocembrin (86) Luteolin (86) Nobiletin (84) Scutellarein (86) Apigenin (86) Chrysin (86) Chrysoeriol (86) Datiscetin (86) 5,4-dihydroxyflavone (86) 6,4-dihydroxyflavone (86) 5,7,2-trihydroxyflavone (86) 3,7,4-trihydroxyflavone (86) 3,6,3,4-tetrahydroxyflavone (86) 5,7-dimethoxyflavone (86) 6,7-dimethoxyflavone (86) 5,7,4-trimethoxyflavone (86) 4-hydroxy-6-methoxyflavone (86) 6-methoxyflavone (86) 7,4′-dihydroxyflavone (86) 6-methoxyluteolin (86) Flavone (86) Herbacetin (86) Isorhamnetin (86) Kaempferol (86) Morin (86) Myricetin (86) Quercetagetin (86) Quercetin (85) Taxifolin (86) Fustin (86) (±)-equol (82) Biochanin A (86) Daidzein (86) Formononetin (82) Genistein (82) Glycitein (82) Isoflavone (82) Prunetin (82) 7-hydroxyisoflavone (82) 7,3,4-trihydroxyisoflavone (82) 6,7,4-trihydroxyisoflavone (82) 7,8,4′-trihydroxyisoflavone (82) 7,4-dimethoxyisoflavone (86) Cyanidin chloride (86) Pelargoninidin chloride (86) Isoxanthohumol (85) Tangeretin (84) 4-hydroxychalcone (86) 2,2′,4′-trihydroxychalcone (86) 3,2-dihydroxychalcone (86) 4,2,5-trihydroxychalcone (86) Butein (86) Chalcone (86) Eriodictyol chalcone (86) Isoliquiritigenin (86) Resveratrol (86) Xanthohumol (85) Procatechuic acid (4) Ferulic acid (4) Vannillic acid (4) Coumestrol (82) Sulfuretin (86) Umbelliferone (60) Silibinin (86) |
| 16 | Arbutin (88) catechole (88) |
| 19 | ND |
| 20 | ND |
| 30 | Epigallocatechin gallate (4) Procatechuic acid (4) Amarogentin (87) |
| 31 | ND |
| 38 | ND |
| 39 | (+)-Catechin (86) (−)-Epicatechin (3) (−)-Epicatechin gallate (83) (−)-Epigallocatechin (4) Epigallocatechin gallate (4) Procyanidin B2g (4) Eriodictyol (86) Hesperitin (86) Homoeriodictyol (86) Liquiritigenin (86) Naringenin (86) Pinocembrin (86) Genkwanin (86) Luteolin (86) Scutellarein (86) Tricetin (86) Apigenin (86) Chrysin (86) Datiscetin (86) 4-hydroxyflavone (86) 5,2-dihydroxyflavone (86) 5,4-dihydroxyflavone (86) 6,4-dihydroxyflavone (86) 5,7,2-trihydroxyflavone (86) 3,7,4-trihydroxyflavone (86) 3,6,3,4-tetrahydroxyflavone (86) 5,7-dimethoxyflavone (86) 6-methoxyflavone (86) 7,4-dihydroxyflavone (86) 6-methoxyluteolin (86) Flavone (86) Fisetin (86) Gossypetin (86) Herbacetin (86) Isorhamnetin (86) Kaempferol (86) Morin (86) Myricetin (86) Quercetagetin (86) Taxifolin (86) Fustin (86) (±)-equol (82) acetylgenistin (82) Biochanin A (86) Daidzein (86) Formononetin (82) Genistein (82) Genistin (82) Glycitein (82) Glycitin (82) Malonylgenistin (82) 7-hydroxyisoflavone (82) 7,3,4-trihydroxyisoflavone (82) 6,7,4-trihydroxyisoflavone (82) 7,8,4-trihydroxyisoflavone (82) Cyanidin chloride (86) Pelargoninidin chloride (86) 2,2′,4′-trihydroxychalcone (86) 3,2-dihydroxychalcone (86) 4,2,5′-trihydroxychalcone (86) Butein (86) Chalcone (86) Eriodictyol chalcone (86) Isoliquiritigenin (86) Resveratrol (86) PGG(pentagalloylglucose) (3) Amarogentin (87) Coumestrol (82) Sulfuretin (86) Silibinin (86) |
| 40 | Isoxanthohumol (85) Xanthohumol (85) |
| 41 | ND |
| 42 | ND |
| 43 | Epigallocatechin gallate (4) Amarogentin (87) |
| 45 | ND |
| 4 | Nobiletin (89) Tangeretin (89) Amarogentin (87) |
| 50 | Amarogentin (81) |
| 60 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules 2024, 14, 234. https://doi.org/10.3390/biom14020234
Osakabe N, Shimizu T, Fujii Y, Fushimi T, Calabrese V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules. 2024; 14(2):234. https://doi.org/10.3390/biom14020234
Chicago/Turabian StyleOsakabe, Naomi, Takafumi Shimizu, Yasuyuki Fujii, Taiki Fushimi, and Vittorio Calabrese. 2024. "Sensory Nutrition and Bitterness and Astringency of Polyphenols" Biomolecules 14, no. 2: 234. https://doi.org/10.3390/biom14020234
APA StyleOsakabe, N., Shimizu, T., Fujii, Y., Fushimi, T., & Calabrese, V. (2024). Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules, 14(2), 234. https://doi.org/10.3390/biom14020234