PAK1-Dependent Regulation of Microtubule Organization and Spindle Migration Is Essential for the Metaphase I–Metaphase II Transition in Porcine Oocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Chemicals
2.2. Oocytes Collection and Culture
2.3. Immunofluorescence Staining
2.4. Immunoblotting
2.5. Co-Immunoprecipitation (Co-IP)
2.6. Experimental Design
2.6.1. Effects of IPA-3 Treatment during the MI–MII Transition
2.6.2. Effects of Dithiothreitol (DTT) on IPA-3 Inhibition
2.7. Statistical Analysis
3. Results
3.1. Expression and Localization of p-PAK1
3.2. IPA-3 Treatment Caused Oocytes Maturation Failure and Cell Cycle Arrest during the MI–MII Transition
3.3. IPA-3 Treatment Perturbed Cytoskeletal Dynamic of Porcine Oocytes during the MI–MII Stage
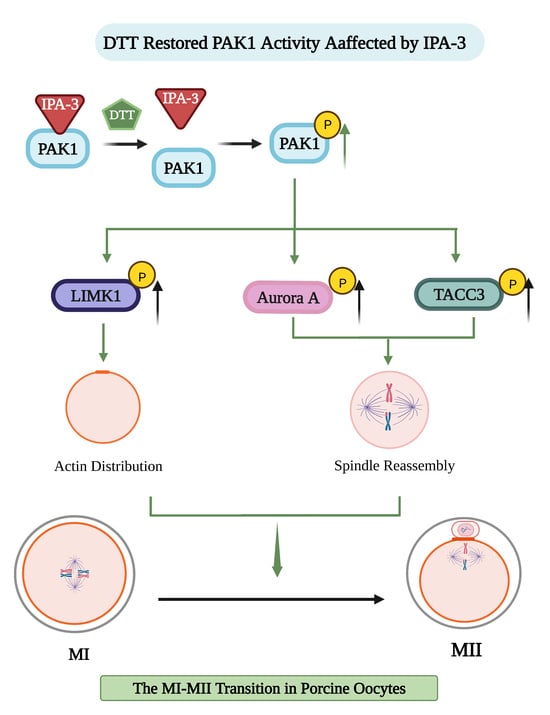
3.4. DTT Recovered the Oocytes’ Maturation and Normal Cytoskeletal Dynamics
3.5. IPA-3 Treatment Decreased the Activity of Aurora A, TACC3, and LIMK during the MI–MII Transition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, H.; Nicholas, N.S.; Wells, C.M. Role of p-21-activated kinases in cancer progression. Int. Rev. Cell Mol. Biol. 2014, 309, 347–387. [Google Scholar] [CrossRef]
- Banerjee, M.; Worth, D.; Prowse, D.M.; Nikolic, M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr. Biol. 2002, 12, 1233–1239. [Google Scholar] [CrossRef]
- Eby, J.J.; Holly, S.P.; van Drogen, F.; Grishin, A.V.; Peter, M.; Drubin, D.G.; Blumer, K.J. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr. Biol. 1998, 8, 967. [Google Scholar] [CrossRef] [PubMed]
- Cotteret, S.; Chernoff, J. Pak GITs to Aurora-A. Dev. Cell 2005, 5, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Whalley, H.J.; Porter, A.P.; Diamantopoulou, Z.; White, G.R.M.; Castañeda-Saucedo, E.; Malliri, A. Cdk1 phosphorylates the Rac activator Tiam1 to activate centrosomal Pak and promote mitotic spindle formation. Nat. Commun. 2015, 6, 7437. [Google Scholar] [CrossRef] [PubMed]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef]
- McGarry, D.J.; Castino, G.; Lilla, S.; Carnet, A.; Kelly, L.; Micovic, K.; Zanivan, S.; Olson, M.F. MICAL1 activation by PAK1 mediates actin filament disassembly. Cell Rep. 2022, 41, 111442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gratzke, C.; Tamalunas, A.; Wiemer, N.; Ciotkowska, A.; Rutz, B.; Waidelich, R.; Strittmatter, F.; Liu, C.; Stief, C.G.; et al. P21-Activated Kinase Inhibitors FRAX486 and IPA3: Inhibition of Prostate Stromal Cell Growth and Effects on Smooth Muscle Contraction in the Human Prostate. PLoS ONE 2016, 4, e153312. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Liu, K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol. Cell. Endocrinol. 2014, 1, 480–487. [Google Scholar] [CrossRef]
- Solc, P.; Schultz, R.M.; Motlik, J. Prophase I arrest and progression to metaphase I in mouse oocytes: Comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol. Hum. Reprod. 2010, 16, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, I.; Terret, M.-E.; Verlhac, M.-H. Meiotic spindle assembly and chromosome segregation in oocytes. J. Cell Biol. 2016, 215, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Azoury, J.; Lee, K.W.; Georget, V.; Rassinier, P.; Leader, B.; Verlhac, M.-H. Spindle Positioning in Mouse Oocytes Relies on a Dynamic Meshwork of Actin Filaments. Curr. Biol. 2008, 18, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, X.; Liu, X.; Cao, Y.; Chen, D.; Wang, Q.; Du, J.; Weng, J.; Ma, W. p21-activated kinase 1 activity is required for histone H3 Ser10 phosphorylation and chromatin condensation in mouse oocyte meiosis. Reprod. Fertil. Dev. 2017, 29, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Lin, S.-L.; Qi, S.-T.; Sun, S.-C.; Wang, Y.-P.; Schatten, H. PAK1 regulates spindle microtubule organization during oocyte meiotic maturation. Front. Biosci. 2010, 2, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Y.; Wang, W.; Chu, Y.; Lin, Q.; Rui, R.; Li, Q.; Ju, S. PAK1 Is Involved in the Spindle Assembly during the First Meiotic Division in Porcine Oocytes. Int. J. Mol. Sci. 2023, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, Y.; Galiani, D.; Nevo, N.; Dekel, N.; Ben-Eliezer, I. Appropriate expression of Ube2C and Ube2S controls the progression of the first meiotic division. FASEB J. 2015, 29, 4670–4681. [Google Scholar] [CrossRef]
- Brieño-Enríquez, M.A.; Moak, S.L.; Holloway, J.K.; Cohen, P.E. NIMA-related kinase 1 (NEK1) regulates meiosis I spindle assembly by altering the balance between α-Adducin and Myosin X. PLoS ONE 2017, 12, e0185780. [Google Scholar] [CrossRef]
- Blengini, C.S.; Schindler, K. Acentriolar spindle assembly in mammalian female meiosis and the consequences of its perturbations on human reproduction dagger. Biol. Reprod. 2022, 2, 253–263. [Google Scholar] [CrossRef]
- Ju, S.; Peng, X.; Yang, X.; Sozar, S.; Muneri, C.W.; Xu, Y.; Chen, C.; Cui, P.; Xu, W.; Rui, R. Aurora B inhibitor barasertib prevents meiotic maturation and subsequent embryo development in pig oocytes. Theriogenology 2016, 86, 503–515. [Google Scholar] [CrossRef]
- Ma, W.; Hou, Y.; Sun, Q.; Sun, X.; Wang, W. Localization of centromere proteins and their association with chromosomes and microtubules during meiotic maturation in pig oocytes. Reproduction 2003, 126, 731–738. [Google Scholar] [CrossRef]
- Jia, R.X.; Duan, X.; Song, S.J.; Sun, S.C. LIMK1/2 inhibitor LIMKi 3 suppresses porcine oocyte maturation. PeerJ 2016, 4, e2553. [Google Scholar] [CrossRef]
- Deacon, S.W.; Beeser, A.; Fukui, J.A.; Rennefahrt, U.E.; Myers, C.; Chernoff, J.; Peterson, J.R. An Isoform-Selective, Small-Molecule Inhibitor Targets the Autoregulatory Mechanism of p21-Activated Kinase. Chem. Biol. 2008, 15, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.K.; Minden, A. P21 activated kinases: Structure, regulation, and functions. Small GTPases 2014, 5, e28003. [Google Scholar] [CrossRef]
- He, Y.-T.; Yang, L.-L.; Luo, S.-M.; Shen, W.; Yin, S.; Sun, Q.-Y. PAK4 Regulates Actin and Microtubule Dynamics during Meiotic Maturation in Mouse Oocyte. Int. J. Biol. Sci. 2019, 15, 2408–2418. [Google Scholar] [CrossRef]
- Tang, C.-J.C.; Hu, H.-M.; Tang, T.K. NuMA expression and function in mouse oocytes and early embryos. J. Biomed. Sci. 2004, 11, 370–376. [Google Scholar] [CrossRef]
- Breuer, M.; Kolano, A.; Kwon, M.; Li, C.-C.; Tsai, T.-F.; Pellman, D.; Brunet, S.; Verlhac, M.-H. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J. Cell Biol. 2010, 191, 1251–1260. [Google Scholar] [CrossRef]
- Wang, F.; An, G.Y.; Zhang, Y.; Liu, H.L.; Cui, X.S.; Kim, N.H.; Sun, S.C. Arp2/3 complex inhibition prevents meiotic maturation in porcine oocytes. PLoS ONE 2014, 1, e87700. [Google Scholar] [CrossRef]
- Verma, A.; Artham, S.; Alwhaibi, A.; Adil, M.S.; Cummings, B.S.; Somanath, P.R. PAK1 inhibitor IPA-3 mitigates metastatic prostate cancer-induced bone remodeling. Biochem. Pharmacol. 2020, 177, 113943. [Google Scholar] [CrossRef]
- Almonacid, M.; Terret, M.; Verlhac, M.-H. Actin-based spindle positioning: New insights from female gametes. J. Cell Sci. 2014, 127, 477–483. [Google Scholar] [CrossRef]
- Li, H.; Guo, F.; Rubinstein, B.; Li, R. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 2008, 10, 1301–1308. [Google Scholar] [CrossRef]
- Viaud, J.; Peterson, J.R. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol. Cancer Ther. 2009, 8, 2559–2565. [Google Scholar] [CrossRef]
- Zhao, Z.-S.; Lim, J.P.; Ng, Y.-W.; Lim, L.; Manser, E. The GIT-Associated Kinase PAK Targets to the Centrosome and Regulates Aurora-A. Mol. Cell 2005, 20, 237–249. [Google Scholar] [CrossRef]
- May, M.; Schelle, I.; Brakebusch, C.; Rottner, K.; Genth, H. Rac1-dependent recruitment of PAK2 to G2phase centrosomes and their roles in the regulation of mitotic entry. Cell Cycle 2014, 13, 2210–2220. [Google Scholar] [CrossRef]
- Liu, Q.; Ruderman, J.V. Aurora A, mitotic entry, and spindle bipolarity. Proc. Natl. Acad. Sci. USA 2006, 15, 5811–5816. [Google Scholar] [CrossRef]
- Marumoto, T.; Zhang, D.; Saya, H. Aurora-A—A guardian of poles. Nat. Rev. Cancer. 2005, 1, 42–50. [Google Scholar] [CrossRef]
- Lorenzo, C.; Liao, Q.; Hardwicke, M.A.; Ducommun, B. Pharmacological inhibition of Aurora-A but not Aurora-B impairs interphase microtubule dynamics. Cell Cycle 2009, 8, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Huang, C.; Jiao, X.; Wu, D.; Huo, L. The role of TACC3 in mitotic spindle organization. Cytoskeleton 2017, 74, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.G.; Hood, F.E.; Prior, I.A.; Royle, S.J. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 2011, 5, 906–919. [Google Scholar] [CrossRef]
- Burgess, S.G.; Peset, I.; Joseph, N.; Cavazza, T.; Vernos, I.; Pfuhl, M.; Gergely, F.; Bayliss, R. Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly. PLoS Genet. 2015, 11, e1005345. [Google Scholar] [CrossRef]
- Delorme-Walker, V.D.; Peterson, J.R.; Chernoff, J.; Waterman, C.M.; Danuser, G.; DerMardirossian, C.; Bokoch, G.M. Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J. Cell Biol. 2011, 193, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, Z.; Wu, J.; Xie, X.; You, W.; Yu, Z.; Shen, H.; Li, X.; Li, H.; Liu, Y.; et al. Effects of PAK1/LIMK1/Cofilin-mediated Actin Homeostasis on Axonal Injury after Experimental Intracerebral Hemorrhage. Neuroscience 2022, 490, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Alahari, S.K. Nischarin acts via the PAK/LIMK pathway to inhibit breast cancer cell invasion. Cancer Res. 2006, 66, 824. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; He, Y.; Wang, W.; Dai, J.; Li, Q.; Ju, S. PAK1-Dependent Regulation of Microtubule Organization and Spindle Migration Is Essential for the Metaphase I–Metaphase II Transition in Porcine Oocytes. Biomolecules 2024, 14, 237. https://doi.org/10.3390/biom14020237
Peng L, He Y, Wang W, Dai J, Li Q, Ju S. PAK1-Dependent Regulation of Microtubule Organization and Spindle Migration Is Essential for the Metaphase I–Metaphase II Transition in Porcine Oocytes. Biomolecules. 2024; 14(2):237. https://doi.org/10.3390/biom14020237
Chicago/Turabian StylePeng, Lei, Yijing He, Weihan Wang, Jianjun Dai, Qiao Li, and Shiqiang Ju. 2024. "PAK1-Dependent Regulation of Microtubule Organization and Spindle Migration Is Essential for the Metaphase I–Metaphase II Transition in Porcine Oocytes" Biomolecules 14, no. 2: 237. https://doi.org/10.3390/biom14020237
APA StylePeng, L., He, Y., Wang, W., Dai, J., Li, Q., & Ju, S. (2024). PAK1-Dependent Regulation of Microtubule Organization and Spindle Migration Is Essential for the Metaphase I–Metaphase II Transition in Porcine Oocytes. Biomolecules, 14(2), 237. https://doi.org/10.3390/biom14020237