Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extraction
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid Content
2.3.3. Total Anthocyanin Content
2.3.4. Total Proanthocyanidin Content
2.3.5. LC-MS/MS Analysis of Specific Polyphenols
2.3.6. Sugars and Organic Acid Analyses
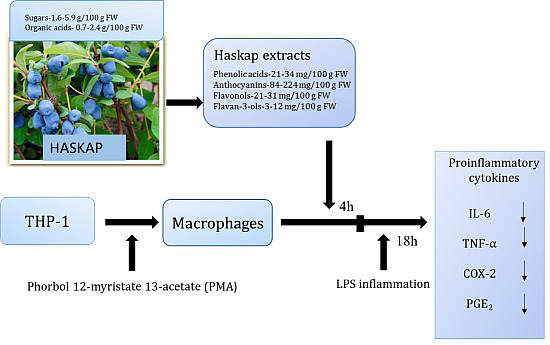
2.4. Cell Culture
2.4.1. Measurement of Cell Viability
2.4.2. Measurement of Nitric Oxide
2.4.3. Measurement of COX-2 Activity
2.4.4. Measurement of IL-6 and TNF-α
2.4.5. Measurement of PGE2
2.5. Statistical Analysis
3. Results and Discussion
3.1. Qualitative Phenolic Composition
| Growing Location | Cultivar | Total Phenolics (mg GAE/100 g FW) | Total Flavonoids (mg QE/100 g FW) | Total Proanthocyanidins (mg CE/100 g FW) | Total Anthocyanins (mg CGE/100 g FW) |
|---|---|---|---|---|---|
| LaHave farm | BL | 755.9 ± 9.4 d,e,f | 1156.6 ± 121.7 b,c | 13.2 ± 1.0 c | 163.0 ± 10.1 c |
| BR | 1154.1 ± 59.7 a | 1582.8 ± 140.5 a | 16.3 ± 0.9 c | 314.0 ± 2.7 a | |
| TN | 952.9 ± 18.7 b,c | 1260.3 ± 69.0 b,c | 16.0 ± 1.0 c | 234.4 ± 2.6 b | |
| IG | 884.3 ± 25.0 c,d | 1327.0 ± 12.1 b,c | 14.4 ± 0.5 c | 246.9 ± 13.7 b | |
| Kentville | LC-12 | 849.2 ± 28.3 c,d | 1035.5 ± 86.7 b,c | 37.2 ± 0.9 b | 164.9 ± 5.4 c |
| LC-13 | 796.8 ± 5.2 d | 997.0 ± 32.0 b,c | 41.0 ± 3.6 b | 142.5 ± 9.1 c | |
| LC-16 | 664.9 ± 51.4 e,f | 900.7 ± 16.6 c,d | 52.3 ± 2.3 a | 70.2 ± 2.2 d | |
| LC-23 | 658.1 ± 0.4 e,f | 956.3 ± 11.0 b,c | 34.2 ± 0.8 b | 120.2 ± 1.5 c,d | |
| LC-47 | 634.4 ± 29.9 f | 916.5 ± 65.2 c,d | 47.0 ± 0.4 a,b | 133.4 ± 1.2 c | |
| Saskatchewan | SAS-IG | 790.3 ± 68.4 d | 1128.5 ± 54.2 c,d | 19.6 ± 2.0 c | 246.3 ± 2.8 b |
| SAS-TN | 1015.3 ± 78.2 a,b | 1428.4 ± 35.1 a,b | 38.5 ± 0.9 b | 303.2 ± 5.5 a |
3.1.1. Total Flavonoid Content
3.1.2. Total Anthocyanin Content
3.1.3. Total Proanthocyanidin Content
3.1.4. LC-MS/MS Composition of Haskap Berry Extract

| LaHave Farm | Kentville | Saskatchewan | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | BR | BL | TN | IG | LC-12 | LC-13 | LC-16 | LC-23 | LC-47 | SAS-IG | SAS-TN |
| Phenolic acids | |||||||||||
| Chlorogenic acid | 25.6 ± 3.6 | 23.1 ± 2.4 | 26.0 ± 2.4 | 22.4 ± 2.5 | 29.7 ± 0.6 | 32.7 ± 3.2 | 23.0 ± 0.2 | 23.9 ± 1.3 | 28.6 ± 0.1 | 20.7 ± 1.1 | 33.8 ± 0.7 |
| Caffeic acid | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | ND | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Total | 25.8 | 23.2 | 26.2 | 22.5 | 29.8 | 32.8 | 23.0 | 24.0 | 28.7 | 20.8 | 33.9 |
| Flavan-3-ols | |||||||||||
| EGC | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Catechin | 2.5 ± 0.2 | 1.7 ± 0.3 | 3.5 ± 0.2 | 2.9 ± 0.3 | 2.1 ± 0.1 | 2.1 ± 0.3 | 3.9 ± 0.1 | 5.4 ± 0.0 | 2.1 ± 0.1 | 2.2 ± 0.1 | 3.4 ± 0.2 |
| Epicatechin | 1.2 ± 0.1 | 1.7 ± 0.4 | 0.7 ± 0.1 | 1.5 ± 0.1 | 4.5 ± 0.1 | 5.8 ± 0.5 | 7.1 ± 0.1 | 6.2 ± 0.1 | 1.5 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| EGCG | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Total | 3.9 | 3.7 | 4.5 | 5.1 | 6.8 | 8.3 | 11.2 | 12.0 | 4.0 | 3.4 | 4.6 |
| Flavonols | |||||||||||
| Q. galactoside | ND | 0.1 ± 0.0 | ND | ND | 0.1 ± 0.0 | 0.1 ± 0.0 | ND | ND | ND | ND | ND |
| Q. glucoside | 3.6 ± 0.2 | 4.2 ± 0.8 | 1.4 ± 0.2 | 2.8 ± 0.2 | 7.3 ± 0.8 | 4.4 ± 0.6 | 3.7 ± 0.1 | 3.8 ± 0.1 | 3.7 ± 0.1 | 2.7 ± 0.1 | 4.0 ± 0.2 |
| Q. arabinoside | 2.9 ± 0.3 | 2.0 ± 0.3 | 1.4 ± 0.2 | 1.1 ± 0.1 | 11.9 ± 0.8 | 9.2 ± 0.8 | 11.4 ± 0.3 | 7.3 ± 0.4 | 10.0 ± 0.0 | 1.0 ± 0.0 | 2.9 ± 0.1 |
| Q. rhamnoside | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 2.2 ± 0.2 | 1.2 ± 0.2 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Q | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Q. rutinoside | 24.3 ± 1.2 | 16.7 ± 6.0 | 19.9 ± 3.0 | 21.6 ± 0.6 | 8.5 ± 0.3 | 6.2 ± 0.2 | 7.7 ± 0.6 | 10.9 ± 1.1 | 6.7 ± 0.1 | 19.5 ± 1.3 | 20.4 ± 1.3 |
| Total | 31.1 | 23.5 | 22.9 | 25.8 | 30.1 | 21.2 | 23.3 | 22.4 | 20.9 | 23.5 | 27.5 |
| Dihydrochalcones | |||||||||||
| Phloridzin | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.25 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| Anthocyanins | |||||||||||
| C-3-gluc | 170.0 ± 10.1 | 140.8 ± 8.3 | 104.7 ± 10.3 | 143.9 ± 1.5 | 147.2 ± 5.6 | 107.7 ± 2.1 | 67.7 ± 2.7 | 103.4 ± 3.1 | 107.2 ± 0.1 | 138.8 ± 8.2 | 164.3 ± 6.2 |
| D-3-glu | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| P-3-gluc | 13.7 ± 0.9 | 9.5 ± 0.5 | 6.7 ± 0.7 | 8.2 ± 0.3 | 9.9 ± 0.1 | 4.3 ± 0.2 | 3.9 ± 0.3 | 7.6 ± 0.3 | 4.9 ± 0.3 | 7.8 ± 0.3 | 14.7 ± 0.4 |
| D-3-rutin | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| C-3-rutin | 39.2 ± 2.3 | 26.1 ± 7.5 | 64.9 ± 9.5 | 38.6 ± 2.5 | 19.5 ± 1.0 | 17.4 ± 0.6 | 11.8 ± 1.3 | 32.0 ± 0.2 | 22.7 ± 0.2 | 31.9 ± 1.3 | 34.6 ± 1.1 |
| C-3-galact | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.8 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| Total | 223.9 | 177.1 | 177.5 | 191.2 | 177.2 | 129.9 | 83.7 | 143.2 | 135.2 | 178.9 | 214.5 |
| Total phenolics by LCMS | 285.1 a | 227.8 c,d | 231.29 c,d | 244.87 b,c | 244.25 b,c | 192.5 f | 209.12 c,d,e | 201.92 d,e | 188.9 f | 226.9 c,d | 280.6 a |
3.1.5. Sugar and Organic Acid Profile
3.2. Inhibition of Inflammatory Markers by Haskap Berry Extract


| Parameters | COX-2 | TNF-α | IL-6 | PGE2 | NO |
|---|---|---|---|---|---|
| Phenolics | −0.781, 0.003 | −0.935, 0.000 | −0.896, 0.000 | −0.026, 0.936 | 0.324, 0.304 |
| Flavonoids | −0.742, 0.006 | −0.781, 0.003 | −0.723, 0.008 | −0.186, 0.563 | 0.264, 0.406 |
| Anthocyanins | −0.728, 0.007 | −0.831, 0.001 | −0.629, 0.028 | 0.005, 0.988 | 0.327, 0.300 |
| Proanthocyanidins | −0.211, 0.511 | −0.290, 0.360 | −0.298, 0.348 | 0.459, 0.134 | 0.084, 0.796 |
3.3. Correlation between Phenolics and Inflammatory Parameters
4. Conclusions
Acknowledgements
Author Contributions
Appendix

Conflict of Interests
References
- Diplock, A.T.; Charleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Vina-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80, S77–S112. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, F.; Parmentier, M.; Hirani, N.; Drost, E.; Rahman, I.; Donaldson, K.; MacNee, W. LPS stimulation of IL-8 release is inhibited by thiol antioxidant at the transcriptional level in THP-1 macrophage cells. Am. J. Respir. Crit. Care Med. 2000, 161, 1319–1327. [Google Scholar]
- Mehta, J.L.; Rasouli, N.; Sinha, A.K.; Molavi, B. Oxidative stress in diabetes, a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int. J. Biochem. Cell. Biol. 2006, 38, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals; metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory non-communicable diseases. J. Allergy Clin. Immunol. 2013, 131, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Canadian Health Measures Survey: Metabolic Syndrome in Canadians. Available online: http://www.statcan.gc.ca/pub/82-625-x/2012001/article/11735-eng.htm#n1 (accessed on 13 February 2014).
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Nair, S.; Robinson, R. Studies in Natural Products Chemistry; Ur Rahman, A., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2014; Volume 42, pp. 229–266. [Google Scholar]
- Bors, B. Breeding of Lonicera caerulea L. for saskatchewan and Canada. In Proceedings of the 1st Virtual International Scientific Conference on Lonicera caerulea L., Saskatoon, SK, Canada, 23 March–23 April 2009; pp. 88–98.
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Tanaka, T.; Onoe, K.; Ohno, S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide induced inflammation in vitro and in vivo. Exp. Eye. Res. 2006, 82, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Yu, L.J.; Bhullar, K.S.; Bors, B. Haskap (Lonicera caerulea): A new berry crop with high antioxidant capacity. Can. J. Plant Sci. 2012, 92, 1311–1317. [Google Scholar] [CrossRef]
- Palikova, I.; Valentova, K.; Oborna, I.; Ulrichova, J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J. Agric. Food Chem. 2009, 57, 6584–6589. [Google Scholar]
- Zdarilova, A.; Svobodova, A.R.; Chytilova, K.; Simanek, V.; Ulrichova, J. Polyphenolic fraction of Lonicera caerulea L. fruits reduced oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food Chem. Toxicol. 2010, 48, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Plekhanova, M.N. Blue honeysuckle (Lonicera caerulea L.)—A new commercial berry crop for temperate climate: Genetic resources and breeding. Acta Hortic. 2000, 538, 159–164. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Tech. Metall. 2005, 40, 255–260. [Google Scholar]
- Ratnasooriya, C.; Rupasinghe, H.P.V.; Jamieson, A. Juice quality and polyphenol concentration of fresh fruits and pomace of selected Nova Scotia-grown grape cultivars. Can. J. Plant Sci. 2010, 90, 193–205. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Erkan, N.; Yasmin, A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic-enriched apple skin extract. J. Agric. Food Chem. 2010, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Reznicek, V.; Mlcek, J.; Jurikova, T.; Balik, J.; Sochor, J.; Kramarova, D. Antioxidant and radical oxygen species scavenging activities of 12 cultivars of blue honeysuckle fruit. Hortic. Sci. 2011, 38, 63–70. [Google Scholar]
- Petrova, V.P. Biochimija Dikorastuščich Plodovo—Jagodnych Rastenij(In Ukraine), 1st ed.; Golovnoe Izdateľstvo Objedenija: Kijev, Ukraine, 1986; pp. 260–266. [Google Scholar]
- Wojdylo, A.; Jauregui, P.N.N.; Carbonell-Barrachina, A.; Oszmianski, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Orincak, J.; Matuskovic, J.; Jurcak, S. Possibilities of Species Lonicera caerulea in Utilization of the Secondary Metabolism in Food and Pharmaceutical Processing, 1st ed.; SPU: Nitra, Slovak, 2003; pp. 210–219. [Google Scholar]
- Plekhanova, M.N.; Streltsyna, S.A.; Rostova, N.S. Phenolic compounds in berries of Lonicera subsect. Caerulea species. Plant Res. 1993, 29, 16–25. [Google Scholar]
- Andersen, O.M.; Jordheim, M. The anthocyanins. In Flavonoids Chemistry, Biochemistry and Applications, 4th ed.; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–552. [Google Scholar]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Jurikova, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical determination of antioxidant potential of some less common fruit species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef]
- Bakowska, A.M.; Marianchuk, M.; Kolodziejczyk, P. Survey of bioactive components in Western Canadian berries. Can. J. Physiol. Pharmacol. 2007, 85, 1139–1152. [Google Scholar]
- Tsuda, T.; Horia, F.; Osawa, T. Cyanidin 3-O-β-d-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J. Nutr. Sci. Vitaminol. 2002, 48, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Retterstol, L.; Laake, P.; Paur, I.; Kjolsrud-Bohn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [PubMed]
- Leiss, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.H. Rutin suppresses atopic dermatitis and allergic contact dermatitis. Exp. Biol. Med. 2012, 238, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ma, Y.; Liu, D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm. Res. 2013, 30, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; So, H.S.; Moon, B.S.; Youn, M.J.; Kim, H.J.; Shin, Y.I.; Moon, S.K.; Song, M.S.; Choi, K.Y.; Song, J.; et al. Sasim attenuates LPS-induced TNF-alpha production through the induction of HO-1 in THP-1 differentiated macrophage-like cells. J. Ethnopharmacol. 2008, 119, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, Y.; Zhang, H.; Gao, X.; Wilson, E.; Zimmer, W.; Abbott, L.; Zhang, C. Role of MCP-1 in tumor necrosis factor-α-induced endothelial dysfunction in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1208–H1216. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Gilmour, P.S.; Jimenez, L.A.; MacNee, W. Oxidative stress and TNF alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: Potential mechanism in gene transcription in lung inflammation. Mol. Cell Biochem. 2002, 234–235, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Anti-atherosclerotic effects of fruit bioactive compounds: A review of current scientific evidence. Can. J. Plant Sci. 2012, 92, 407–419. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Porawski, M.; Alonso, M.; Marroni, N.; Collado, P.S.; Gonzalez-Gallego, J. Quercetin decreases oxidative stress, NF-kappa β activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J. Nutr. 2005, 135, 2299–2304. [Google Scholar] [PubMed]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Mullebner, A.; Paier-Pourani, J.; Banerjee, A.; Miller, I.; Lauterbock, L.; Duvigneau, J.C.; Skulachev, V.P.; Redl, H.; Kozlov, A.V. Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid. Redox Signal. 2015, 22, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Jones, Q.R.D.; Warford, J.; Rupasinghe, H.P.V.; Robertson, G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012, 33, 602–610. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupasinghe, H.P.V.; Boehm, M.M.A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.R. Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent. Biomolecules 2015, 5, 1079-1098. https://doi.org/10.3390/biom5021079
Rupasinghe HPV, Boehm MMA, Sekhon-Loodu S, Parmar I, Bors B, Jamieson AR. Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent. Biomolecules. 2015; 5(2):1079-1098. https://doi.org/10.3390/biom5021079
Chicago/Turabian StyleRupasinghe, H. P. Vasantha, Mannfred M. A. Boehm, Satvir Sekhon-Loodu, Indu Parmar, Bob Bors, and Andrew R. Jamieson. 2015. "Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent" Biomolecules 5, no. 2: 1079-1098. https://doi.org/10.3390/biom5021079
APA StyleRupasinghe, H. P. V., Boehm, M. M. A., Sekhon-Loodu, S., Parmar, I., Bors, B., & Jamieson, A. R. (2015). Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent. Biomolecules, 5(2), 1079-1098. https://doi.org/10.3390/biom5021079