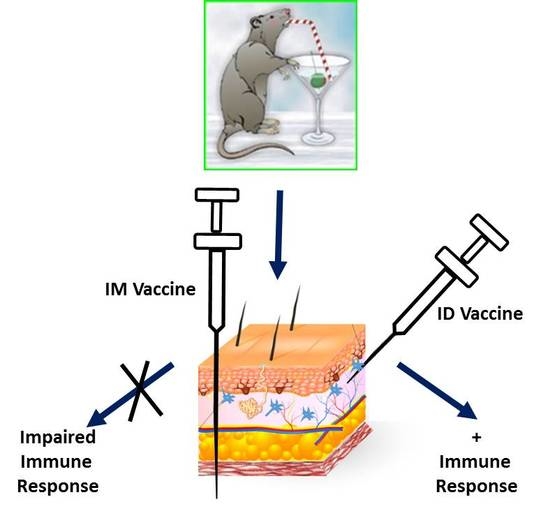
Skin Immunization Obviates Alcohol-Related Immune Dysfunction
Abstract
:1. Introduction
2. Results
2.1. EtOH Levels Are Significantly Less in Skin than in Blood
| Diet | LD EtOH | MC EtOH | LD Control | MC Control |
|---|---|---|---|---|
| Serum EtOH (%) | 0.1380 ± 0.0251 a,b | 0.0244 ± 0.0099 a,c | 0.0007 ± 0.0006 b | 0.0005 ± 0.0003 c |
| Skin EtOH (%) | 0.0070 ± 0.0017 d | 0.0036 ± 0.0014 e | 0.0006 ± 0.0002 d | 0.0005 ± 0.0003 e |
2.2. Alcohol Feeding Protocols Differentially Induce Steatohepatitis and Oxidative Stress

2.3. Increases in Myeloid Derived Suppressor Cell (MDSC) Populations Correlate with Alcohol Induced Oxidative Stress

2.4. Skin Immunization Obviates Alcohol Associated DTH Inhibition

2.5. Skin Immunization Overcomes Alcohol Induced Inhibition of CTL Induction

2.6. Skin Immunization Obviates Alcohol Induced Inhibition of Antigen-Specific IgG Induction

3. Discussion
4. Experimental Section
4.1. Feeding Regimes
4.2. Immunization Methods
4.3. Serum and Tissue Characterization
4.4. Liver Biochemical Assays
4.5. 4HNE Immunohistochemistry
4.6. Myeloid Derived Suppressor Cells (MDSC) and Regulatory T Cells (Treg)
4.7. Delayed Type Hypersensitivity (DTH)
4.8. In Vivo Cytotoxic T Lymphocyte Killing Assay (CTL)
4.9. Antibody Measurements
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meyerholz, D.K.; Edsen-Moore, M.; McGill, J.; Coleman, R.A.; Cook, R.T.; Legge, K.L. Chronic alcohol consumption increases the severity of murine influenza virus infections. J. Immunol. 2008, 181, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Lin, C.C.; Mo, L.R.; Chang, C.Y.; Perng, D.S.; Hsu, C.C.; Lo, G.H.; Chen, Y.S.; Yen, Y.C.; Hu, J.T.; et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J. Hepatol. 2013, 58, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Bagby, G.J.; Nelson, S. Biomedical consequences of alcohol use disorders in the hiv-infected host. Curr. HIV Res. 2014, 12, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Chaves, S.S.; Perez, A.; Aragon, D.; Bandyopadhyay, A.; Bennett, N.; Fowler, B.; Hancock, E.; Lynfield, R.; McDonald-Hamm, C.; et al. Heavy alcohol use as a risk factor for severe outcomes among adults hospitalized with laboratory-confirmed influenza, 2005–2012. Infection 2014, 42, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Parlet, C.P.; Kavanaugh, J.S.; Horswill, A.R.; Schlueter, A.J. Chronic ethanol feeding increases the severity of Staphylococcus aureus skin infections by altering local host defenses. J Leukoc. Biol. 2015, 97, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.M.; Dobard, E.; Zhang, P.; Nelson, S. Alcohol exacerbates murine pulmonary tuberculosis. Infecti. Immun. 2004, 72, 2556–2563. [Google Scholar] [CrossRef]
- Shellito, J.E.; Quan Zheng, M.; Ye, P.; Ruan, S.; Shean, M.K.; Kolls, J. Effect of alcohol consumption on host release of interleukin-17 during pulmonary infection with Klebsiella pneumoniae. Alcohol. Clin. Exp. Res. 2001, 25, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Grau, I.; Ardanuy, C.; Calatayud, L.; Schulze, M.H.; Liñares, J.; Pallares, R. Smoking and alcohol abuse are the most preventable risk factors for invasive pneumonia and other pneumococcal infections. Int. J. Infect. Dis. 2014, 25, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.; Roselle, G.A.; Lybecker, L.A.; Marshall, L.E.; Grossman, C.J.; Myre, S.A.; Weesner, R.E.; Morgan, D.D. Hepatitis B vaccination. Response of alcoholic with and without liver injury. Dig. Dis. Sci. 1988, 33, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Nalpas, B.; Thepot, V.; Driss, F.; Pol, S.; Courouce, A.M.; Saliou, P.; Berthelot, P. Secondary immune response to hepatitis B virus vaccine in alcoholics. Alcohol. Clin. Exp. Res. 1993, 17, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Benin, A.L.; O’Brien, K.L.; Watt, J.P.; Reid, R.; Zell, E.R.; Katz, S.; Donaldson, C.; Parkinson, A.; Schuchat, A.; Santosham, M.; et al. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in navajo adults. J. Infect. Dis. 2003, 188, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Porretta, E.; Happel, K.I.; Teng, X.S.; Ramsay, A.; Mason, C.M. The impact of alcohol on BCG-induced immunity against Mycobacterium tuberculosis. Alcohol. Clin. Exp. Res. 2012, 36, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rosman, A.S.; Basu, P.; Galvin, K.; Lieber, C.S. Efficacy of a high and accelerated dose of hepatitis B vaccine in alcoholic patients: A randomized clinical trial. Am. J. Med. 1997, 103, 217–222. [Google Scholar] [CrossRef]
- De Maria, N.; Idilman, R.; Colantoni, A.; van Thiel, D.H. Increased effective immunogenicity to high-dose and short-interval hepatitis B virus vaccination in individuals with chronic hepatitis without cirrhosis. J. Viral Hepat. 2001, 8, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Nagafuchi, S.; Kashiwagi, S.; Okada, K.; Anzai, K.; Nakamura, M.; Nishimura, Y.; Sasazuki, T.; Niho, Y. Reversal of nonresponders and postexposure prophylaxis by intradermal hepatitis B vaccination in japanese medical personnel. JAMA 1991, 265, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Dahmen, A.; Herzog-Hauff, S.; Bocher, W.O.; Galle, P.R.; Lohr, H.F. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology 2000, 31, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, B.; Liard, C. Transcutaneous and intradermal vaccination. Hum. Vaccine 2011, 7, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Pasala, S.; Grant, K. Could moderate alcohol intake be recommended to improve vaccine responses? Expert Rev. Vaccines 2014, 13, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.E.; Mendenhall, C.L.; Roselle, G.A.; Grossman, C.J. Cell-mediated immune responses associated with short term alcohol intake: Time course and dose dependency. Alcohol. Clin. Exp. Res. 1989, 13, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.L.; Theus, S.A.; Roselle, G.A.; Grossman, C.J.; Rouster, S.D. Biphasic in vivo immune function after low- versus high-dose alcohol consumption. Alcohol 1997, 14, 255–260. [Google Scholar] [CrossRef]
- Lau, A.H.; Szabo, G.; Thomson, A.W. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Jendrzejewski, J.L. Chronic ethanol ingestion alters xenobiotic absorption through the skin: Potential role of oxidative stress. Food Chem. Toxicol. 2008, 46, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Edsen-Moore, M.R.; Fan, J.; Ness, K.J.; Marietta, J.R.; Cook, R.T.; Schlueter, A.J. Effects of chronic ethanol feeding on murine dendritic cell numbers, turnover rate, and dendropoiesis. Alcohol. Clin. Exp. Res. 2008, 32, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.J.; Fan, J.; Wilke, W.W.; Coleman, R.A.; Cook, R.T.; Schlueter, A.J. Chronic ethanol consumption decreases murine langerhans cell numbers and delays migration of langerhans cells as well as dermal dendritic cells. Alcohol. Clin. Exp. Res. 2008, 32, 657–668. [Google Scholar] [CrossRef] [PubMed]
- D’Souza El-Guindy, N.B.; Kovacs, E.J.; de Witte, P.; Spies, C.; Littleton, J.M.; de Villiers, W.J.; Lott, A.J.; Plackett, T.P.; Lanzke, N.; Meadows, G.G. Laboratory models available to study alcohol-induced organ damage and immune variations: Choosing the appropriate model. Alcohol. Clin. Exp. Res. 2010, 34, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.M.; Starkey, J.R.; Meadows, G.G. Toxicity of chronic high alcohol intake on mouse natural killer cell activity. Res. Commun. Chem. Pathol. Pharmacol. 1988, 59, 245–258. [Google Scholar] [PubMed]
- Cook, R.T.; Schlueter, A.J.; Coleman, R.A.; Tygrett, L.; Ballas, Z.K.; Jerrells, T.R.; Nashelsky, M.B.; Ray, N.B.; Haugen, T.H.; Waldschmidt, T.J. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion—Absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol. Clin. Exp. Res. 2007, 31, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. The feeding of alcohol in liquid diets: Two decades of applications and 1982 update. Alcohol. Clin. Exp. Res. 1982, 6, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Bode, J.C. Activation of the innate immune system and alcoholic liver disease: Effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res. 2005, 29, 166S–171S. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Thakur, V.; Pritchard, M.T.; McMullen, M.R.; Nagy, L.E. Regulation of kupffer cell activity during chronic ethanol exposure: Role of adiponectin. J. Gastroenterol. Hepatol. 2006, 21, S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Jendrzejewski, J.L.; Charron, A.R. Potential mechanisms by which a single drink of alcohol can increase transdermal absorption of topically applied chemicals. Toxicology 2007, 235, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, E.; Husby, G.; Williams, R.C., Jr.; Strickland, R.G. Liver disease—A prominent cause of serum ige elevation. Clin. Exp. Immunol. 1976, 23, 444–450. [Google Scholar] [PubMed]
- Wang, H.J.; Gao, B.; Zakhari, S.; Nagy, L.E. Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 2012, 32, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Wang, H.; Chan, Y.-L.; Li, T.-L.; Bauer, B.A.; Hsia, S.; Wang, C.-H.; Huang, J.-S.; Wang, H.-M.; Yeh, K.-Y.; Huang, T.-H.; et al. Reduction of splenic immunosuppressive cells and enhancement of anti-tumor immunity by synergy of fish oil and selenium yeast. PLoS ONE 2013, 8, e52912. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Bando, Y.; Xiao, S.; Yang, K.; Anderson, A.C.; Kuchroo, V.K.; Khoury, S.J. Cd11b+ly−6c(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007, 179, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Akbar, S.M.; Abe, M.; Hiasa, Y.; Onji, M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin. Exp. Immunol. 2011, 166, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, Y.; Falo, L.D., Jr.; Hui, K.M.; Huang, L. Regression of human mammary adenocarcinoma by systemic administration of a recombinant gene encoding the hflex-trail fusion protein. Mol. Ther. 2001, 3, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; University of Pittsburgh, Pittsburgh, PA, USA. Unpublished work. 2009.
- Lau, A.H.; Abe, M.; Thomson, A.W. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J. Leukoc. Biol. 2006, 79, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Edsen-Moore, M.R.; Turner, L.E.; Cook, R.T.; Legge, K.L.; Waldschmidt, T.J.; Schlueter, A.J. Mechanisms by which chronic ethanol feeding limits the ability of dendritic cells to stimulate T-cell proliferation. Alcohol. Clin. Exp. Res. 2011, 35, 47–59. [Google Scholar] [CrossRef]
- Parlet, C.P.; Schlueter, A.J. Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol. Clin. Exp. Res. 2013, 37, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Heinz, R.; Waltenbaugh, C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol. Clin. Exp. Res. 2007, 31, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Effect of biphenyl dimethyl dicarboxylate on the cellular and nonspecific immunotoxicity by ethanol in mice. Biol. Pharm. Bull. 2000, 23, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Nielsen, N.H.; Linneberg, A. The association between alcohol consumption and contact sensitization in danish adults: The glostrup allergy study. Br. J. Dermatol. 2008, 158, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Gesien, A.; Wands, J.R. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J. Immunol. 1997, 159, 5107–5113. [Google Scholar] [PubMed]
- Encke, J.; Wands, J.R. Ethanol inhibition: The humoral and cellular immune response to hepatitis C virus ns5 protein after genetic immunization. Alcohol. Clin. Exp. Res. 2000, 24, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Aloman, C.; Gehring, S.; Wintermeyer, P.; Kuzushita, N.; Wands, J.R. Chronic ethanol consumption impairs cellular immune responses against HCV ns5 protein due to dendritic cell dysfunction. Gastroenterology 2007, 132, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Amakawa, R.; Kaisho, T.; Hemmi, H.; Tajima, K.; Uehira, K.; Ozaki, Y.; Tomizawa, H.; Akira, S.; Fukuhara, S. Interferon-alpha and interleukin-12 are induced differentially by toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 2002, 195, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.J.; Song, J.Y.; Park, J.W.; Yeon, J.E.; Byun, K.S.; Lee, C.H.; Cho, H.I.; Kim, T.G.; Kim, W.J. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine 2006, 24, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.J.; Rettmann, N.A.; Dieperink, E.W.; Durfee, J.; Aqel, B. Antibody response to hepatitis B vaccine in substance use disorder patients. Drug Alcohol. Depend. 2010, 107, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Arnou, R.; Icardi, G.; de Decker, M.; Ambrozaitis, A.; Kazek, M.P.; Weber, F.; van Damme, P. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine 2009, 27, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P.; Martin, P. Intradermal vs. intramuscular vaccine against hepatitis B infection in dialysis patients: A meta-analysis of randomized trials. J. Viral Hepat. 2011, 18, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.; Moore, C.; Li, S.D.; Aziz, A.; Kakar, A.; Dosanjh, A.; Beesla, A.; Murphy, L.; van Thiel, D.H. Efficacy of high-dose intra-dermal hepatitis B virus vaccine in previous vaccination non-responders with chronic liver disease. Dig. Dis. Sci. 2012, 57, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Morelon, E.; Pouteil Noble, C.; Daoud, S.; Cahen, R.; Goujon-Henry, C.; Weber, F.; Laurent, P.E.; Kaiserlian, D.; Nicolas, J.F. Immunogenicity and safety of intradermal influenza vaccination in renal transplant patients who were non-responders to conventional influenza vaccination. Vaccine 2010, 28, 6885–6890. [Google Scholar] [CrossRef] [PubMed]
- Latif, O.; Peterson, J.D.; Waltenbaugh, C. Alcohol-mediated polarization of type 1 and type 2 immune responses. Front. Biosci. 2002, 7, a135–a147. [Google Scholar] [CrossRef] [PubMed]
- Eken, A.; Ortiz, V.; Wands, J.R. Ethanol inhibits antigen presentation by dendritic cells. Clin. Vaccine Immunol. 2011, 18, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, T.J.; Cook, R.T.; Kovacs, E.J. Alcohol and inflammation and immune responses: Summary of the 2005 alcohol and immunology research interest group (AIRIG) meeting. Alcohol 2006, 38, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and its role in the generation of th1 cells. Immunol. Today 1993, 14, 335–338. [Google Scholar] [CrossRef]
- Iclozan, C.; Antonia, S.; Chiappori, A.; Chen, D.-T.; Gabrilovich, D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 2013, 62, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Hogg, A.; Wang, Y.; Frey, B.; Yu, H.; Xia, Z.; Venzon, D.; McKinnon, K.; Smedley, J.; Gathuka, M.; et al. Vaccine-induced myeloid cell population dampens protective immunity to SIV. J. Clin. Investig. 2014, 124, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meadows, G. Chronic alcohol consumption enhances myeloid-derived suppressor cells in b16bl6 melanoma-bearing mice. Cancer Immunol. Immunother. 2010, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, K.K.; Samak, G.; Shukla, P.K.; Mir, H.; Gangwar, R.; Manda, B.; Isse, T.; Kawamoto, T.; Salaspuro, M.; Kaihovaara, P.; et al. Aldh2 deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol. Clin. Exp. Res. 2015, 39, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Roychowdhury, S.; McMullen, M.R.; Stavitsky, A.B.; Nagy, L.E. Complement and alcoholic liver disease: Role of c1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 2010, 139, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Larosche, I.; Choumar, A.; Fromenty, B.; Letteron, P.; Abbey-Toby, A.; van Remmen, H.; Epstein, C.J.; Richardson, A.; Feldmann, G.; Pessayre, D.; et al. Prolonged ethanol administration depletes mitochondrial DNA in mnsod-overexpressing transgenic mice, but not in their wild type littermates. Toxicol. Appl. Pharmacol. 2009, 234, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Larregina, A.T.; Morelli, A.E.; Tkacheva, O.; Erdos, G.; Donahue, C.; Watkins, S.C.; Thomson, A.W.; Falo, L.D., Jr. Highly efficient expression of transgenic proteins by naked DNA-transfected dendritic cells through terminal differentiation. Blood 2004, 103, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, C.; Chen, Y.; Burnett, C.; Liu, X.Y.; Downs, S.; Collins, R.D.; Hawiger, J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J. Biol. Chem. 2004, 279, 48434–48442. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; McMullen, M.R.; Pritchard, M.T.; Hise, A.G.; van Rooijen, N.; Medof, M.E.; Stavitsky, A.B.; Nagy, L.E. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 2009, 49, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Liu, Z.X.; Lane, T.; Dennert, G. Ip-10 and mig facilitate accumulation of T cells in the virus-infected liver. Cell Immunol. 2002, 219, 48–56. [Google Scholar] [CrossRef]
- Mathers, A.R.; Tckacheva, O.A.; Janelsins, B.M.; Shufesky, W.J.; Morelli, A.E.; Larregina, A.T. In vivo signaling through the neurokinin 1 receptor favors transgene expression by langerhans cells and promotes the generation of th1- and tc1-biased immune responses. J. Immunol. 2007, 178, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.A.; Falkner, D.; Plowey, J.; Larregina, A.T.; Falo, L.D. DNA immunisation: Altering the cellular localisation of expressed protein and the immunisation route allows manipulation of the immune response. Vaccine 2004, 22, 447–456. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brand, R.M.; Stottlemyer, J.M.; Cline, R.A.; Donahue, C.; Behari, J.; Falo Jr., L.D. Skin Immunization Obviates Alcohol-Related Immune Dysfunction. Biomolecules 2015, 5, 3009-3028. https://doi.org/10.3390/biom5043009
Brand RM, Stottlemyer JM, Cline RA, Donahue C, Behari J, Falo Jr. LD. Skin Immunization Obviates Alcohol-Related Immune Dysfunction. Biomolecules. 2015; 5(4):3009-3028. https://doi.org/10.3390/biom5043009
Chicago/Turabian StyleBrand, Rhonda M., John Mark Stottlemyer, Rachel A. Cline, Cara Donahue, Jaideep Behari, and Louis D. Falo Jr. 2015. "Skin Immunization Obviates Alcohol-Related Immune Dysfunction" Biomolecules 5, no. 4: 3009-3028. https://doi.org/10.3390/biom5043009
APA StyleBrand, R. M., Stottlemyer, J. M., Cline, R. A., Donahue, C., Behari, J., & Falo Jr., L. D. (2015). Skin Immunization Obviates Alcohol-Related Immune Dysfunction. Biomolecules, 5(4), 3009-3028. https://doi.org/10.3390/biom5043009