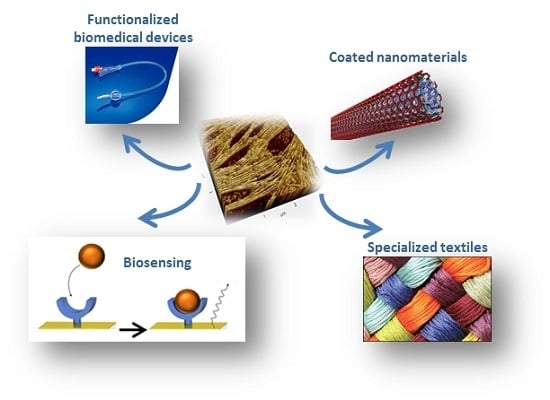
Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins
Abstract
:1. Introduction
2. Metal and Metalloid Functionalization
3. Plastic Functionalization
4. Carbon Nanotubes and 2D Materials Functionalization
5. Functionalization of Other Materials
5.1. Biosensing and Biomedical Applications
5.2. Textiles Finishing Processes
5.3. Biocatalytic Transformations
6. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—From bacteria to humans. Trends. Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Loquet, A.; Saupe, S. Diversity of Amyloid Motifs in NLR Signaling in Fungi. Biomolecules 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science 1994, 264, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.; Wösten, H.A.B.; Scholtmeijer, K. Creating surface properties using a palette of hydrophobins. Materials (Basel) 2010, 3, 4607–4625. [Google Scholar] [CrossRef]
- Whiteford, J.R.; Spanu, P.D. Hydrophobins and the interactions between fungi and plants. Mol. Plant Pathol. 2002, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Kwan, A.H.Y.; Templeton, M.D.; Beever, R.E.; Mackay, J.P. Structural analysis of hydrophobins. Micron 2008, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.H.Y.; Winefield, R.D.; Sunde, M.; Matthews, J.M.; Haverkamp, R.G.; Templeton, M.D.; Mackay, J.P. Structural basis for rodlet assembly in fungal hydrophobins. Proc. Natl. Acad. Sci. USA 2006, 103, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Macindoe, I.; Kwan, A.H.; Ren, Q.; Morris, V.K.; Yang, W.; Mackay, J.P.; Sunde, M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc. Natl. Acad. Sci. USA 2012, 109, E804–E811. [Google Scholar] [CrossRef] [PubMed]
- Houmadi, S.; Rodriguez, R.D.; Longobardi, S.; Giardina, P.; Fauré, M.C.; Giocondo, M.; Lacaze, E. Self-Assembly of Hydrophobin Protein Rodlets Studied with Atomic Force Spectroscopy in Dynamic Mode. Langmuir 2012, 28, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Lo, V.; Ren, Q.; Pham, C.; Morris, V.; Kwan, A.; Sunde, M. Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability. Nanomaterials 2014, 4, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.B. Hydrophobins: Proteins that self assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Morris, V.K.; Kwan, A.H.; Sunde, M. Analysis of the Structure and Conformational States of DewA Gives Insight into the Assembly of the Fungal Hydrophobins. J. Mol. Biol. 2013, 425, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.L.L.; Rey, A.; Lo, V.; Soulès, M.; Ren, Q.; Meisl, G.; Knowles, T.P.J.; Kwan, A.H.; Sunde, M. Self-assembly of MPG1, a hydrophobin protein from the rice blast fungus that forms functional amyloid coatings, occurs by a surface-driven mechanism. Sci. Rep. 2016, 6, 25288. [Google Scholar] [CrossRef] [PubMed]
- Gandier, J.-A.; Langelaan, D.N.; Won, A.; O’Donnell, K.; Grondin, J.L.; Spencer, H.L.; Wong, P.; Tillier, E.; Yip, C.; Smith, S.P.; Master, E.R. Characterization of a Basidiomycota hydrophobin reveals the structural basis for a high-similarity Class I subdivision. Sci. Rep. 2017, 7, 45863. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B.; Scholtmeijer, K. Applications of hydrophobins: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Am. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Santhiya, D.; Burghard, Z.; Greiner, C.; Jeurgens, L.P.H.; Subkowski, T.; Bill, J. Bioinspired Deposition of TiO2 Thin Films Induced by Hydrophobins. Langmuir 2010, 26, 6494–6502. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Throm, T.; Gutt, B.; Strunk, T.; Hoffmann, M.; Seebach, E.; Mühlberg, L.; Brocher, J.; Gotterbarm, T.; Wenzel, W.; et al. Engineering hydrophobin DewA to generate surfaces that enhance adhesion of human but not bacterial cells. Acta Biomater. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Rea, I.; Armenante, A.; Giardina, P.; Giocondo, M.; Rendina, I. Self-assembled biofilm of hydrophobins protects the silicon surface in the KOH wet etch process. Langmuir 2007, 23, 7920–7922. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Rea, I.; De Tommasi, E.; Rendina, I.; Rotiroti, L.; Giocondo, M.; Longobardi, S.; Armenante, A.; Giardina, P. Bioactive modification of silicon surface using self-assembled hydrophobins from Pleurotus ostreatus. Eur. Phys. J. E 2009, 30, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Canham, L.K. Properties of Porous Silicon; Canham, L., Ed.; The Institution of Electrical Engineers: London, UK, 1997. [Google Scholar]
- De Stefano, L.; Rea, I.; Giardina, P.; Armenante, A.; Rendina, I. Protein-Modified Porous Silicon Nanostructures. Adv. Mater. 2008, 20, 1529–1533. [Google Scholar] [CrossRef]
- Longobardi, S.; Gravagnuolo, A.M.; Rea, I.; De Stefano, L.; Marino, G.; Giardina, P. Hydrophobin-coated plates as matrix-assisted laser desorption/ionization sample support for peptide/protein analysis. Anal. Biochem. 2014, 449, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, S.; Gravagnuolo, A.M.; Funari, R.; Della Ventura, B.; Pane, F.; Galano, E.; Amoresano, A.; Marino, G.; Giardina, P. A simple MALDI plate functionalization by Vmh2 hydrophobin for serial multi-enzymatic protein digestions. Anal. Bioanal. Chem. 2015, 407, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.; Cicatiello, P.; Deininger, L.; Clench, M.R.; Marino, G.; Giardina, P.; Langenburg, G.; West, A.; Marshall, P.; Sears, V.; et al. A proteomic approach for the rapid, multi-informative and reliable identification of blood. Analyst 2016, 141, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, B.; Rea, I.; Caliò, A.; Giardina, P.; Gravagnuolo, A.M.; Funari, R.; Altucci, C.; Velotta, R.; De Stefano, L. Vmh2 hydrophobin layer entraps glucose: A quantitative characterization by label-free optical and gravimetric methods. Appl. Surf. Sci. 2016, 364, 201–207. [Google Scholar] [CrossRef]
- Politi, J.; De Stefano, L.; Longobardi, S.; Giardina, P.; Rea, I.; Methivier, C.; Pradier, C.M.; Casale, S.; Spadavecchia, J. The amphiphilic hydrophobin Vmh2 plays a key role in one step synthesis of hybrid protein-gold nanoparticles. Coll. Surf. B Biointerfaces 2015, 136, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Politi, J.; De Stefano, L.; Rea, I.; Gravagnuolo, A.M.; Giardina, P.; Methivier, C.; Casale, S.; Spadavecchia, J. One-pot synthesis of a gold nanoparticle-Vmh2 hydrophobin nanobiocomplex for glucose monitoring. Nanotechnology 2016, 27, 195701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Li, S.; Xu, H.; Linder, M.B.; Qiao, M. Hydrophilic modification of polystyrene with hydrophobin for time-resolved immunofluorometric assay. Biosens. Bioelectron. 2010, 26, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Li, B.; Wang, H.; Guo, R.; Liang, H.; Qiao, M.; Li, W. Preparing bioactive surface of polystyrene with hydrophobin for trypsin immobilization. Mater. Res. Express 2016, 3, 055402. [Google Scholar] [CrossRef]
- Fokina, O.; Fenchel, A.; Winandy, L.; Fischer, R. Immobilization of LccC Laccase from Aspergillus nidulans on Hard Surfaces via Fungal Hydrophobins. Appl. Environ. Microbiol. 2016, 82, 6395–6402. [Google Scholar] [PubMed]
- Scholtmeijer, K.; Janssen, M.I.; Gerssen, B.; de Vocht, M.L.; van Leeuwen, B.M.; van Kooten, T.G.; Wösten, H.A.B.; Wessels, J.G.H. Surface modifications created by using engineered hydrophobins. Appl. Environ. Microbiol. 2002, 68, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.I.; van Leeuwen, M.B.M.; Scholtmeijer, K.; van Kooten, T.G.; Dijkhuizen, L.; Wösten, H.A.B. Coating with genetic engineered hydrophobin promotes growth of fibroblasts on a hydrophobic solid. Biomaterials 2002, 23, 4847–4854. [Google Scholar] [CrossRef]
- Janssen, M.I.; van Leeuwen, M.B.; van Kooten, T.G.; de Vries, J.; Dijkhuizen, L.; Wösten, H.A. Promotion of fibroblast activity by coating with hydrophobins in the β-sheet end state. Biomaterials 2004, 25, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Li, J.; Cannon, G.C.; Morgan, S.E. Nanoscale reduction in surface friction of polymer surfaces modified with SC3 hydrophobin from Schizophyllum commune. Biomacromolecules 2006, 7, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Weickert, U.; Wiesend, F.; Subkowski, T.; Eickhoff, A.; Reiss, G. Optimizing biliary stent patency by coating with hydrophobin alone or hydrophobin and antibiotics or heparin: An in vitro proof of principle study. Adv. Med. Sci. 2011, 56, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.; Niu, B.; Wang, D.; Wang, Z.; Feng, S.; Xu, H.; Kong, D.; Qiao, M. Poly(ɛ-caprolactone) modified with fusion protein containing self-assembled hydrophobin and functional peptide for selective capture of human blood outgrowth endothelial cells. Coll. Surf. B Biointerfaces 2013, 101, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, S.; Pan, Y.; Zhang, Q.; Wang, K.; Song, D.; Wang, X.; Feng, G.; Liu, R.; Xu, H.; et al. Functional Modification of Fibrous PCL Scaffolds with Fusion Protein VEGF-HGFI Enhanced Cellularization and Vascularization. Adv. Healthc. Mater. 2016, 5, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Q.; Zhao, L.; Pan, Y.; Wang, T.; Zhi, D.; Ma, S.; Zhang, P.; Zhao, T.; Zhang, S.; et al. Functional Modification of Electrospun Poly(ε-caprolactone) Vascular Grafts with the Fusion Protein VEGF–HGFI Enhanced Vascular Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 11415–11427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, J.; Chen, Y.; Song, D.; Gao, Z.; Zhang, X.; Bai, Y.; Saris, P.E.J.; Feng, H.; Xu, H.; et al. Design of antibacterial biointerfaces by surface modification of poly (ε-caprolactone) with fusion protein containing hydrophobin and PA-1. Coll. Surf. B Biointerfaces 2017, 151, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Cicatiello, P.; Ricciardelli, A.; Papa, R.; Selan, L.; Dardano, P.; Tilotta, M.; Vrenna, G.; Tutino, M.L.; Giardina, P.; et al. Hydrophobin coating prevents Staphylococcus epidermidis biofilm formation on different surfaces. Biofouling 2017, in press. [Google Scholar]
- Hennig, S.; Rödel, G.; Ostermann, K. Hydrophobin-Based Surface Engineering for Sensitive and Robust Quantification of Yeast Pheromones. Sensors (Basel) 2016, 16, 602. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Pennacchio, A.; Longobardi, S.; Velotta, R.; Giardina, P. Vmh2 hydrophobin as a tool for the development of self-immobilizing enzymes for biosensing. Biotechnol. Bioeng. 2017, 114, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Pennacchio, A.; Cicatiello, P.; Politi, J.; De Stefano, L.; Giardina, P. Rapid and ultrasensitive detection of active thrombin based on the Vmh2 hydrophobin fused to a Green Fluorescent Protein. Biosens. Bioelectron. 2017, 87, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, P.; Dardano, P.; Pirozzi, M.; Gravagnuolo, A.M.; De Stefano, L.; Giardina, P. Self-assembly of two hydrophobins from marine fungi affected by interaction with surfaces. Biotechnol. Bioeng. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Huang, Y.; Li, S.; Feng, S.; Xu, H.; Qiao, M. Characterization and application of hydrophobin-dispersed multi-walled carbon nanotubes. Carbon N. Y. 2010, 48, 2890–2898. [Google Scholar] [CrossRef]
- Gravagnuolo, A.M.; Morales-Narvaez, E.; Longobardi, S.; Da Silva, E.T.; Giardina, P.; Merkoci, A. In situ production of biofunctionalized few-layer defect-free microsheets of graphene. Adv. Funct. Mater. 2015, 25, 2771–2779. [Google Scholar] [CrossRef]
- Kaur, J.; Gravagnuolo, A.M.; Maddalena, P.; Altucci, C.; Giardina, P.; Gesuele, F. Green synthesis of luminescent and defect-free bio-nanosheets of MoS 2 : Interfacing two-dimensional crystals with hydrophobins. RSC Adv. 2017, 7, 22400–22408. [Google Scholar] [CrossRef]
- Yang, W.; Ren, Q.; Wu, Y.-N.; Morris, V.K.; Rey, A.A.; Braet, F.; Kwan, A.H.; Sunde, M. Surface functionalization of carbon nanomaterials by self-assembling hydrophobin proteins. Biopolymers 2013, 99, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Bilewicz, R.; Witomski, J.; Van der Heyden, A.; Tagu, D.; Béatrice Palin, A.; Rogalska, E. Modification of Electrodes with Self-Assembled Hydrophobin Layers. J. Phys. Chem. B 2001, 105, 9772–9777. [Google Scholar] [CrossRef]
- Corvis, Y.; Walcarius, A.; Rink, R.; Mrabet, N.T.; Rogalska, E. Preparing Catalytic Surfaces for Sensing Applications by Immobilizing Enzymes via Hydrophobin Layers. Anal. Chem. 2005, 77, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Li, X.; Li, X.; Feng, X.-Z.; Wang, R.; Wang, C.; Yu, L.; Qiao, M.-Q. Surface modification using a novel type I hydrophobin HGFI. Anal. Bioanal. Chem. 2009, 394, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Rieder, A.; Ladnorg, T.; Wöll, C.; Obst, U.; Fischer, R.; Schwartz, T. The impact of recombinant fusion-hydrophobin coated surfaces on E. coli and natural mixed culture biofilm formation. Biofouling 2011, 27, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Gravagnuolo, A.M.; Morales-Narvaez, E.; Matos, C.R.S.; Longobardi, S.; Giardina, P.; Merkoci, A. On-the-Spot Immobilization of Quantum Dots, Graphene Oxide, and Proteins via Hydrophobins. Adv. Funct. Mater. 2015, 25, 6084–6092. [Google Scholar] [CrossRef]
- Melcher, M.; Facey, S.J.; Henkes, T.M.; Subkowski, T.; Hauer, B. Accelerated Nucleation of Hydroxyapatite Using an Engineered Hydrophobin Fusion Protein. Biomacromolecules 2016, 17, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Opwis, K.; Gutmann, J.S. Surface modification of textile materials with hydrophobins. Text. Res. J. 2011, 81, 1594–1602. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Iordache, O.G.; Mocioiu, A.M.; Nicula, G. Antimicrobial functionalization of textile materials with hydrophobins and Ag/ZnO composite nanopowders. Ind. Text. 2013, 64, 303–312. [Google Scholar]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-Phase Handling of Hydrophobins: Immobilized Hydrophobins as a New Tool To Study Lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, J.; Sun, Y.; Reynolds, M.; Zhang, L.; Han, S.; Liang, S.; Sui, H.; Lin, Y. Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B. Appl. Microbiol. Biotechnol. 2016, 100, 5883–5895. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Subkowski, T.; Bollschweiler, C.; von Vacano, B.; Liu, Y.; Schrepp, W.; Baus, U. Recombinantly produced hydrophobins from fungal analogues as highly surface-active performance proteins. Eur. Biophys. J. 2010, 39, 457–468. [Google Scholar] [CrossRef] [PubMed]





| Application | HFB | Source | Surface | Refs. |
|---|---|---|---|---|
| Medical | eng SC3 1 | S. commune | Teflon | [34,35,36] |
| SC3—RGD 2 | ||||
| eng SC3—RGD 1,2 | ||||
| eng SC3 1 | S. commune | [34,35,36] | ||
| SC3—RGD 2 | ||||
| SC3 | S. commune | [34,35,36] | ||
| SC4 | ||||
| SC3 | S. commune | Polystyrene | [37] | |
| Copolymer of benzoyl-1,4 phenylene and 1,3-phenylene | ||||
| eng DewA 1 | A. nidulans | Plastic biliary stent | [38] | |
| DewA | A. nidulans | Polystyrene | [20] | |
| DewA–RGD 2 | ||||
| DewA–LG3 2 | ||||
| HGFI—TPS 2 | G. frondosa | Polycaprolattone | [39] | |
| HGFI—VGF 2 | G. frondosa | [40] | ||
| HGFI—VGF 2 | G. frondosa | [41] | ||
| HGFI—PA1 2 | G. frondosa | [42] | ||
| Vmh2 | P. ostreatus | Polystyrene | [43] | |
| Pac3 | Acremonium sclerotigenum | |||
| Biosensing | EAS | N. crassa | Polystyrene | [44] |
| EAS-α 2 | ||||
| Vmh2-GST 2 | P. ostreatus | [45] | ||
| Vmh2-GFP 2 | P. ostreatus | [46] | ||
| Immobilization | HGFI | G. frondosa | Polystyrene | [31] |
| rHGFI 3 | G. frondosa | [32] | ||
| DewA–LccC 2 | A. nidulans | [33] | ||
| DewB–LccC 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscitelli, A.; Cicatiello, P.; Gravagnuolo, A.M.; Sorrentino, I.; Pezzella, C.; Giardina, P. Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins. Biomolecules 2017, 7, 45. https://doi.org/10.3390/biom7030045
Piscitelli A, Cicatiello P, Gravagnuolo AM, Sorrentino I, Pezzella C, Giardina P. Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins. Biomolecules. 2017; 7(3):45. https://doi.org/10.3390/biom7030045
Chicago/Turabian StylePiscitelli, Alessandra, Paola Cicatiello, Alfredo Maria Gravagnuolo, Ilaria Sorrentino, Cinzia Pezzella, and Paola Giardina. 2017. "Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins" Biomolecules 7, no. 3: 45. https://doi.org/10.3390/biom7030045
APA StylePiscitelli, A., Cicatiello, P., Gravagnuolo, A. M., Sorrentino, I., Pezzella, C., & Giardina, P. (2017). Applications of Functional Amyloids from Fungi: Surface Modification by Class I Hydrophobins. Biomolecules, 7(3), 45. https://doi.org/10.3390/biom7030045