Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives
Abstract
:1. Introduction
2. Mechanism of Action
3. Milk-Derived Antibacterial Peptides
4. Whey-Derived Antibacterial Peptides
5. Casein-Derived Antibacterial Peptides
6. Lysozyme-Derived Antibacterial Peptides
7. Production of Antibacterial Peptides
7.1. Fermentation by Lactic Acid Bacteria
7.2. Protein Hydrolysis with Extracellular Enzymes (Proteases)
7.3. Antibacterial Peptides Synthesis by Recombinant DNA Method
8. Summary and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Tomioka, H.; Nakagami, H.; Tenma, A.; Saito, Y.; Kaga, T.; Kanamori, T.; Tamura, N.; Tomono, K.; Kaneda, Y.; Morishita, R. Novel anti-microbial peptide SR-0379 accelerates wound healing via the PI3 Kinase/Akt/mTOR pathway. PLoS ONE 2014, 9, e92597. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Moylan, D.C.; Pati, S.K.; Ross, S.A.; Fowler, K.B.; Boppana, S.B.; Sabbaj, S. Breast Milk Human Cytomegalovirus (CMV) Viral Load and the Establishment of Breast Milk CMV-pp65-Specific CD8 T Cells in Human CMV Infected Mothers. J. Infect. Dis. 2017, 216, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Sindayikengera, S.; Xia, W.-S. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J. Zhejiang Univ. Sci. B 2006, 7, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Tacoma, R.; Fields, J.; Ebenstein, D.B.; Lam, Y.-W.; Greenwood, S.L. Characterization of the bovine milk proteome in early-lactation Holstein and Jersey breeds of dairy cows. J. Proteom. 2016, 130, 200–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakala, P.; Vapaatalo, H. Antihypertensive Peptides from Milk Proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M.; Muguerza, B. Peptides with antihypertensive activity from milk and egg proteins. Peptidos antihipertensivos derivados de proteinas de leche y huevo. Nutr. Hosp. 2008, 23, 313–318. [Google Scholar] [PubMed]
- Yamamoto, N.; Takano, T. Antihypertensive peptides derived from milk proteins. Nahrung 1999, 43, 159–164. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-Related Aspects of Milk Proteins. Iran. J. Pharm. Res. 2016, 15, 573–591. [Google Scholar] [PubMed]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Davidson, P.M.; Critzer, F.J.; Taylor, T.M. Naturally occurring antimicrobials for minimally processed foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Berge, G.; Eliassen, L.T.; Camilio, K.A.; Bartnes, K.; Sveinbjrnsson, B.; Rekdal, O. Therapeutic vaccination against a murine lymphoma by intratumoral injection of a cationic anticancer peptide. Cancer Immunol. Immunother. 2010, 59, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Russell, N.J. Food Preservatives, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Della Malva, A.; Marino, R. Bioactive Peptides in Animal Food Products. Foods 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, E1255. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; Lucas, L.R.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Martínez, M.I.; Kok, J. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2002, 42, 91–121. [Google Scholar] [CrossRef] [PubMed]
- Maria-Neto, S.; de Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta 2015, 848, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.D.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Urena, Y.R.; Sanchez, A.; Pereira, R.; Rischka, K.; Kowalik, T.; Vega-Baudrit, J. Extracellular micro and nanostructures forming the velvet worm solidified adhesive secretion. Mater. Res. Express 2017, 4, 125013. [Google Scholar] [CrossRef] [Green Version]
- Bechinger, B.; Lohner, K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A. Antimicrobial peptides from food proteins. Curr. Pharm. Des. 2003, 9, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J.; Schibli, D.J.; Weiguo, J.; Lohmeier-Vogel, E.M.; Epand, R.F.; Epand, R.M. Towards a structure-function analysis of bovine lactoferricin and related tryptophan and arginine containing peptides. Biochem. Cell Biol. 2002, 80, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Antimicrobial peptides generated from milk proteins: A survey and prospects for application in the food industry. A review. Int. J. Dairy Technol. 2010, 63(3), 320–338. [Google Scholar] [CrossRef]
- Hayes, M. Food Proteins and Bioactive Peptides: New and Novel Sources, Characterisation Strategies and Applications. Foods 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.H.A.; El-Shibiny, S. Bioactive peptides of buffalo, camel, goat, sheep, mare, and yak milks and milk products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores, J.D.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lyu, Y.; Bhunia, A.; Narsimhan, G. Antimicrobial peptide segments from soy protein for use in food safety. Abstr. Pap. Am. Chem. S. 2015, 250. [Google Scholar]
- Wessolowski, A.; Bienert, M.; Dathe, M. Antimicrobial activity of arginine- and tryptophan-rich hexapeptides: The effects of aromatic clusters, D-amino acid substitution and cyclization. J. Pept. Res. 2004, 64, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Fowler, R.S.; Martinez, C.; Chen, J.; Tulpinski, J. Activated lactoferrin and fluconazole synergism against Candida albicans and Candida glabrata vaginal isolates. J. Reprod. Med. 2004, 49, 800–807. [Google Scholar] [PubMed]
- Nehete, J.Y.; Bhambar, R.S.; Narkhede, M.R.; Gawali, S.R. Natural proteins: Sources, isolation, characterization and applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Hernandez-Ledesma, B.; Fernandez-Tome, S.; Weinborn, V.; Barile, D.; de Moura Bell, J.M.L.N. Milk proteins, peptides, and oligosaccharides: Effects against the 21st century disorders. Biomed. Res. Int. 2015, 2015, 146840. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Marimuthu, S.K.; Palanisamy, S.; Subbiah, L. Peptide Therapeutics Versus Superbugs: Highlight on Current Research and Advancements. Int. J. Pept. Res. Ther. 2018, 24(1), 19–33. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Graslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, S.; Cardoso, A.L.; Mano, M.; De Lima, M.C.P. Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals 2010, 3, 961–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.H.; Moore, S.E. Dietary proteins in the regulation of food intake and body weight in humans. J. Nutr. 2004, 134, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Kittis, D.; Weiler, K. Bioactive Proteins and Peptides from Food Sources. Applications of Bioprocesses used in Isolation and Recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Lamberti, C.; Purrotti, M.; Mazzoli, R.; Fattori, P.; Barello, C.; Daniel Coïsson, J.; Giunta, C.; Pessione, E. ADI pathway and histidine decarboxylation are reciprocally regulated in Lactobacillus hilgardii ISE 5211: Proteomic evidence. Amino Acids 2011, 41, 517–527. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Chand, R.; Kapila, S. Biofunctional Properties of Bioactive Peptides of Milk Origin. Food Rev. Int. 2009, 25, 28–43. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Biofunctional properties of caseinophosphopeptides in the oral cavity. Caries Res. 2012, 46, 234–267. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, R.; Rana, S. Bioactive peptides: A review. Int. J. Bioautom. 2011, 15, 223–250. [Google Scholar]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy. Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eichler, J.; Pischetsrieder, M. Virtual screening of a milk peptide database for the identification of food-derived antimicrobial peptides. Mol. Nutr. Food Res. 2015, 59, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- McCann, K.B.; Shiell, B.J.; Michalski, W.P.; Lee, A.; Wan, J.; Roginski, H. Isolation and characterisation of a novel antibacterial peptide from bovine αs1-casein. Int. Dairy J. 2006, 16, 316–323. [Google Scholar] [CrossRef]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.J.M.; Senesi, S.; van Dissel, J.T.; Nibbering, P.H. Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species. Antimicrob. Agents. Ch. 2003, 47(1), 262–267. [Google Scholar] [CrossRef]
- Hill, R.D.; Lahov, E.; Givol, D. A rennin-sensitive bond in alpha-s1 b-casein. J. Dairy Res. 1974, 41, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Recio, I.; Visser, S. Identification of two distinct antibacterial domains within the sequence of bovine αS2-casein. Biochim. Biophys. Acta 1999, 1428, 314–326. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hiratani, T.; Uchida, K.; Yamaguchi, H. Antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin. J. Infect. Chemother. 1996, 1, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Malkoski, M.; Dashper, S.G.; O’Brien-Simpson, N.M.; Talbo, G.H.; Macris, M.; Cross, K.J. Kappacin a novel antimicrobial peptide from bovine milk. Antimicrob. Agents Chemother. 2001, 45, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Exposito, I.; Quiros, A.; Amigo, L.; Recio, I. Casein hydrolysates as a source of antimicrobial, antioxidative and antihypertensive peptides. Le Lait 2007, 87, 241–249. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Takase, M.; Tomita, M. Lactoferricin derived from milk protein lactoferrin. Curr. Pharm. Des. 2003, 9, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.M.; Vogel, H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 2009, 91, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zucht, H.D.; Raida, M.; Adermann, K.; Magert, H.J.; Forssman, W.G. Casocidin-I: A casein αS2-derived peptide exhibits antibacterial activity. FEBS Lett. 1995, 372, 185–188. [Google Scholar] [CrossRef]
- Reiter, B.; Oram, J.D. Bacterial inhibitors in milk and other biological fluids. Nature 1967, 216, 328–330. [Google Scholar] [CrossRef]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [PubMed]
- Hettiarachchy, S.N.; Sato, K.; Marshall, M.R.; Kannan, A. Bioactive Food Proteins and Peptides: Applications in Human Health; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gigli, I. Milk Proteins from Structure to Biological Properties and Health Aspects; INTech Open Publications: Rijeka, Croatia, 2016. [Google Scholar]
- Holt, C. The milk salts and their interaction with casein. In Advanced Dairy Chemistry; Fox, P.F., Ed.; Chapman & Hall: London, UK, 1997; pp. 233–256. [Google Scholar]
- Jabbari, A.; Suárez-Fariñas, M.; Dewell, S.; Krueger, J.G. Transcriptional profiling of psoriasis using RNA-seq reveals previously unidentified differentially expressed genes. J. Investig. Dermatol. 2012, 132, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Takase, M.; Bellami, W.; Shimamura, S. A review: The active peptide of lactoferrin. Acta Paediatr. Jpn. 1994, 36, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Barrett, E.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Evaluation of an antimicrobial ingredient prepared from a Lactobacillus acidophilus caseinfermentate against Enterobacter sakazakii. J. Food Prot. 2009, 72, 340–346. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Rivas, L.; Burgess, C.M.; Fanning, S.; Duffy, G. Inhibition of verocytotoxigenic Escherichia coli by antimicrobial peptides caseicin A and B and the factors affecting their antimicrobial activities. Int. J. Food Microbiol. 2012, 153, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Norberg, S.; O’Connor, P.M.; Stanton, C.; Ross, R.P.; Hill, C.; Fitzgerald, G.F. Altering the composition of caseicins A and B as a means of determining the contribution of specific residues to antimicrobial activity. Appl. Environ. Microbiol. 2011, 77, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, V. Milk Proteins-derived antibacterial peptides as novel functional food ingredients. Ann. Biol. Res. 2012, 3, 2520–2526. [Google Scholar]
- Tidona, F.; Criscione, A.; Guastella, A.N.; Zuccaro, A.; Bordonaro, S.; Marletta, D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009, 8, 315–340. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Kansal, V.K. Purification, characterization, antibacterial activity and N-terminal sequencing of buffalo-milk lysozyme. J Dairy Res. 2002, 69, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.N.; Jing, W.; Schibli, D.J.; Trinh, T.; Park, I.Y.; Kim, S.C.; Vogel, H.J. The interactions of antimicrobial peptides derived from lysozyme with model membrane systems. Biochim. Biophys. Acta 2005, 1668, 175–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Exposito, I.; Minervini, F.; Amigo, L.; Recio, I. Identification of antibacterial peptides from bovine kappa-casein. J. Food Prot. 2006, 69, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of peptides exhibiting biological activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteotytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Antimicrobial peptides generated from milk proteins: A survey and prospects for application in the food industry—A review. Int. J. Dairy Technol. 2010, 63, 320–338. [Google Scholar] [CrossRef]
- Martinenko, N.I.; Yagodinskaya, S.G.; Adhundov, A.A.; Charyev, K.C.; Khumedov, O. Contet of trace element, copper, manganese molybdenum in culture of chal and camel milk and their clinical significance. Dairy Sci. 1977, 40, 824. [Google Scholar]
- Muhialdin, B.J.; Hassan, Z.; Abu Bakar, F.; Saari, N. Identification of Antifungal Peptides Produced by Lactobacillus plantarum IS10 Grown in the MRS broth. Food Control 2016, 59, 27–30. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Papagianni, M.; Filiousis, G.; Ambrosiadis, I.; Koidis, P. Pediocin SA-1, an antimicrobial peptide from Pediococcus acidilactici NRRL B5627: Production conditions, purification and characterization. Bioresour. Technol. 2008, 99, 5384–5390. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Milioni, C.; Martínez, B.; Degl’Innocenti, S.; Turchi, B.; Fratini, F.; Cerri, D.; Fischetti, R. A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Sonsa-Ard, N.; Rodtong, S.; Chikindas, M.L.; Yongsawatdigul, J. Characterization of bacteriocin produced by Enterococcus faecium CN-25 isolated from traditionally Thai fermented fish roe. Food Control 2015, 54, 308–316. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, R.; Carlson, T.; Schaffner, D.W. Unmasking seafood mislabeling in US markets: DNA barcoding as a unique technology for food authentication and quality control. Food Control 2015, 56, 71–76. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. Antimicrobial peptides of dairy proteins: From fundamental to applications. Food Rev. Int. 2014, 30, 134–154. [Google Scholar] [CrossRef]
- Cheigh, C.; Pyun, Y. Nisin biosynthesis and its properties. Biotechnol. Lett. 2005, 27, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- De Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Vessoni Penna, T.C. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 1996, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-López, A.; Martín-Belloso, O. Use of nisin and other bacteriocins for preservation of dairy products. Int. Dairy J. 2008, 18, 329–343. [Google Scholar] [CrossRef]
- Kjos, M.; Nes, I.F.; Diep, D.B. Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology 2009, 155, 2949–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed Elsayed, E.; Abdel Fattah Farid, M.; Ali El Enshasy, H. Improvement in natamycin production by Streptomyces natalensis with the addition of short-chain carboxylic acids. Process Biochem. 2013, 48, 1831–1838. [Google Scholar] [CrossRef]
- Scientific Opinion on the use of natamycin (E 235) as a food additive; Natamycin, Pimaricin, Antibiotics, E 235, CAS 7681-93-8, Antibiotic Resistance; EFSA: Parma, Italy, 2009.
- Lahov, E.; Regelson, W. Antibacterial and immunostimulating casein-derived substances from milk: Casecidin, isracidin peptides. Food Chem. Toxicol. 1996, 34, 131–145. [Google Scholar] [CrossRef]
- Chantaysakorn, P.; Richter, R.L. Antimicrobial properties of pepsin-digested lactoferrin added to carrot juice and filtrate of carrot juice. J. Food Prot. 2000, 63, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Vilcinskas, A. Antimicrobial Peptides: The Ancient Arm of the Human Immune System. Virulence 2010, 5, 440–464. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Zhao, X. Epigenetic marks: Regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front. Genet. 2015, 28, 302. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R.; Musicaro, R.; Pan, L.; Schacter, D.L. Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychol. Aging 2010, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Szwajkowska, M.; Wolanciuk, A.; Wolanciuk, J. Bovine milk protein as the source of bioactive peptides influencing the consumers’ immune system. Anim. Sci. Pap. Rep. 2011, 29, 269–280. [Google Scholar]
- Waqhu, F.H.; Gopi, L.; Ramteke, P.; Nizami, B.; Idicula-Thomas, S. CAMP: Collection of sequences and structures of antimicrobial peptide. Nucleic Acids Res. 2014, 42, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Geetha, R.; Sathian, C.T.; Prasad, V.; Gleeja, V.L. Efficacy of purified antimicrobial peptides from lactic acid bacteria against bovine mastitis pathogens. Asian J. Dairy Food Res. 2015, 34, 259–264. [Google Scholar] [CrossRef]
- Pingitore, E.; Salvucci, V.E.; Sesma, F.; Macías, N.M.E. Different strategies for purification of antimicrobial peptides from Lactic Acid Bacteria (LAB). Trends Appl. Microbiol. 2007, 21, 557–568. [Google Scholar]
- Kozak, W.; Trzpil, M.R.; Dobrzanski, W.T. Preliminary observations on the influence of proflavin, ethidium bromide, and elevated temperature on the production of the antibiotic nisin by Streptococcus lactis strains. Bull. Acad. Pol. Sci. 1973, 21, 811–817. [Google Scholar]
- Chassy, B.M. A gentle method for the lysis of oral Streptococci. Biochem. Biophys. Res. Commun. 1976, 68, 603–608. [Google Scholar] [CrossRef]
- Daveyand, G.P.; Richardson, B.C. Purification and Some Properties of Diplococcin from Streptococcus cremoris 346. Appl. Environ. Microbiol. 1981, 41, 84–89. [Google Scholar]
- Hastings, J.W.; Sailer, M.; Johnson, K.; Roy, K.L.; Vederas, J.C.; Stiles, M.E. Characterization of leucocin A-UAL187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 1991, 173, 7491–7500. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bajaj, R.; Mann, B. Characterization of Antimicrobial and Antioxidative Peptides Synthesized by L. rhamnosus C6 Fermentation of Milk. Int. J. Pept. Res. Ther. 2018, 24, 309–321. [Google Scholar] [CrossRef]
- Liepke, C.; Zucht, H.D.; Forssmann, W.G.; Standker, L. Purification of novel peptide antibiotics from human milk. J. Chromatogr. B 2001, 752, 369–377. [Google Scholar] [CrossRef]
- Kussmann, M.; Nordhoff, E.; Rahbek-Nielsen, H.; Haebel, S.; Rossel-Larsen, M.; Jakobsen, L.; Gobom, J.; Mirgorodskaya, E.; Kroll-Kristensen, A.; Palm, L.; et al. Matrix-assisted Laser Desorption/Ionization Mass Spectrometry Sample Preparation Techniques Designed for Various Peptide and Protein Analytes. J. Mass Spect. 1997, 32, 593–601. [Google Scholar] [CrossRef]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, A.; Hempel, F.; Li, A.; Linne, U.; Maier, U.G.; Reetz, M.T.; Geyer, A. Hinge-Type Dimerization of Proteins by a Tetracysteine Peptide of High Pairing Specificity. Biochemistry 2018, 57, 3658–3664. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.F.; Cardoso, J.M.D.O.; Reis, L.E.S.; Vieira, J.F.; Mathias, F.A.S.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Ruiz, J.C.; Resende, D.D.M.; Reis, A.B. Peptide Vaccines for Leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Soundrarajan, N.; Cho, H.-S.; Ahn, B.; Choi, M.; Thong, L.M.; Choi, H.; Cha, S.-Y.; Kim, J.-H.; Park, C.-K.; Seo, K.; et al. Green fluorescent protein as a scaffold for high efficiency production of functional bacteriotoxic proteins in Escherichia coli. Sci. Rep. 2016, 6, 20661. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, M.; Fazeli, A.; Niazi, A.; Ghabooli, M. Recombinant expression of LFchimera antimicrobial peptide in a plant-based expression system and its antimicrobial activity against clinical and phytopathogenic bacteria. Biotechnol. Biotechnol. Equip. 2018, 32, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Boga, S.; Bouzada, D.; Pena, D.G.; Lopez, M.V.; Vazquez, M.E. Sequence-Specific DNA Recognition with Designed Peptides. Eur. J. Org. Chem. 2018, 249–261. [Google Scholar] [CrossRef]
- Lepage, P.; Heckel, C.; Humbert, S.; Stahl, S.; Rautmann, G. Recombinant Technology as an Alternative to Chemical Peptide-Synthesis—Expression and Characterization of HIV-1 Rev Recombinant Peptides. Anal. Biochem. 1993, 213, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Espita, P.J.P.; De Fátima, N.F.S.; Coimbra, J.S.R.; Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2009, 5, 1447–1464. [Google Scholar] [CrossRef]
- Feng, J. Tyrosine phosphorylation by Src within the cavity of the adenine nucleotide translocase 1 regulates ADP/ATP exchange in mitochondria. Am. J. Physiol. Cell Physiol. 2010, 298, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Stefanidakis, M.; Ning, L.; Van Lint, P.; Nyman-Huttunen, H.; Libert, C.; Itohara, S.; Mishina, M.; Rauvala, H.; Gahmberg, C.G. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 2007, 178, 687–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X. Identification of a novel nuclear-localized adenylate kinase 6 from Arabidopsis thaliana as an essential stem growth factor. Plant Physiol. Biochem. 2012, 61, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Prak, K.; Utsumi, S. Production of a bioactive peptide (IIAEK) in Escherichia coli using soybean proglycinin A1ab1b as a carrier. J. Agric. Food Chem. 2009, 13, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
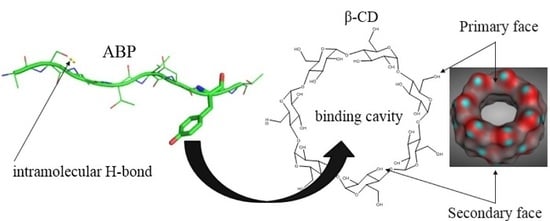
- Shityakov, S.; Salmas, R.E.; Durdagi, S.; Salvador, E.; Papai, K.; Yanez-Gascon, M.J.; Perez-Sanchez, H.; Puskas, I.; Roewer, N.; Forster, C.; et al. Characterization, in vivo Evaluation, and Molecular Modeling of Different Propofol-Cyclodextrin Complexes to Assess Their Drug Delivery Potential at the Blood-Brain Barrier Level. J. Chem. Inf. Model. 2016, 56, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, C.; Sawicki, W.; Marianowski, P.; Benchaib, M.; Czyba, J.C.; Guerin, J.F. Cyclodextrin enhances spermicidal effects of magainin-2-amide. Contraception 2000, 62, 99–103. [Google Scholar] [CrossRef]
- Shityakov, S.; Forster, C. Pharmacokinetic delivery and metabolizing rate of nicardipine incorporated in hydrophilic and hydrophobic cyclodextrins using two-compartment mathematical model. Sci. World J. 2013, 2013, 131358. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Broscheit, J.; Forster, C. Alpha-Cyclodextrin dimer complexes of dopamine and levodopa derivatives to assess drug delivery to the central nervous system: ADME and molecular docking studies. Int. J. Nanomed. 2012, 7, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Sobkoa, A.A.; Kotovaa, E.A.; Antonenkoa, Y.N.; Zakharov, S.D.; Cramerb, W.A. Effect of lipids with different spontaneous curvature on the channel activity of colicin E1: Evidence in favor of a toroidal pore. FEBS Lett. 2004, 576, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.L.; Mitchell, D.C.; Litman, B.J. Manipulation of cholesterol levels in rod disk membranes by methyl-β-cyclodextrin. Effects on receptor activation. J. Biol. Chem. 2002, 277, 20139–20145. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.-S.; Rittner, H.L.; Roewer, N.; Sohajda, T.; Shityakov, S.; Brack, A.; Broscheit, J.-A. A Novel Approach for the Control of Inflammatory Pain: Prostaglandin E2 Complexation by Randomly Methylated beta-Cyclodextrins. Anesth. Analg. 2017, 124, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, R. New insights into the advantages of ammonium as a winemaking nutrient. Int. J. Food Microbiol. 2014, 177, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, P.; Zhongtang, Y. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar]


| ABPs | Circulatory System | Nervous System | Immune System | Gastrointestinal Tract | Functional Peptide |
| Antihypertensive peptides | Opioid peptides | Immunomodulation peptides | Regulatory and enzyme inhibitors | Sensory peptides | |
| Antithrombotic peptides | Antibacterial peptides | Celiac toxicity | Antioxidative peptides | ||
| Microelement-binding peptides | Surface active peptides |
| ABP | MIC | Pathogen |
|---|---|---|
| αs2-casein f(151–181) | 15.6 μg/mL | Bacillus subtilis ATCC6051, |
| 16.2 μM (62.5 μg/mL) | Escherichia coli NEB5α and E. coli, ATCC25922 | |
| αs2-casein f(182–207) | 2.7 μM (8.6 μg/mL) | B. subtilis ATCC6051, |
| 21.4 μM (68.8 μg/mL) | E. coli NEB5α, | |
| Lactoferrin | 125 mg/mL | E. coli, |
| 250 mg/mL | Salmonella typhimurium, | |
| 125 mg/mL | Salmonella enteritidis, | |
| 500 mg/mL | Citrobacter freundii, | |
| 2.5 mg/mL | Candida albicans |
| ABP | Production | Inhibition | References |
|---|---|---|---|
| Isracidin αs1 f(1–23) | Chymosin digestion | Several microorganisms in vivo and in vitro | [64] |
| Lactoferrin B f(18–36) and f(17–41/42) | Enzymatic digestion (pepsin and chymosin) | Some Gram (+) and Gram (−) bacteria | [65,66] |
| Lactoferricin f(17–41) | Enzymatic digestion (pepsin and chymosin) | Some Gram (+) and Gram (−) bacteria, viruses, fungi, and parasites | [65,67] |
| Lf f(268–284) | Enzymatic digestion (pepsin and chymosin) | B. subtilis, E. coli, P. aeruginosa | [68] |
| αs2 casein f(183–207) | Digestion with pepsin | Some Gram (+) and Gram (−) bacteria | [66] |
| κ-casein f(106–169) (kappacin) | Digestion with chymosin | S. mutans, E. coli | [69] |
| κ-casein f(18–24) and f(30–32) and f(139–146) | Digestion with pepsin | Some Gram (+) and Gram (−) bacteria | [70] |
| Lf f(1–48) and f(1–47) | Digestion with pepsin | M. flavus | [70] |
| α–La f(1–5) and f(17–31) and f(61–68) | Digestion with chymotrypsin | Some Gram (+) Gram (−) bacteria | [71] |
| B–Lg f(15–20), f(25–40), f(78–83) and f(92–100) | Digestion with trypsin | Some Gram (+) and Gram (−) bacteria | [71] |
| Production Method | Production | Scale |
|---|---|---|
| Fermentation | Not precise | Laboratory and industrial |
| Protein hydrolysis | Not precise | Limited to laboratory |
| Recombinant DNA | Large ABPs (>150 amino acids) | Laboratory and industrial |
| Organic synthesis | Medium-size ABPs | Laboratory and industrial |
| Derivative Antibacterial Peptides | Parental Compound | Expression System | Inhibited Growth | Reference |
|---|---|---|---|---|
| Lactoferricin B-W10 (LfcinB-W10), | Lactoferricin Lf-(f17–41) | E. coli BL21 (DE3). | S. aureus ATCC25923 | [134] |
| Lfcin B15-W4,10 | Lactoferricin Lf-(f17–31) | E. coli BL21 (DE3). | S. aureus ATTC25923 | [135] |
| LFT33 | Bovine lactoferricin and thanatin (an inducible insect antibacterial peptide) | E. coli BL21 | Significant antibacterial activity compared to parental compound | [136] |
| Lactophoricin | Residues 113–135 of proteose-peptone (component 3) | E. coli C41 (DE3) | Not mentioned | [137] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives. Biomolecules 2018, 8, 110. https://doi.org/10.3390/biom8040110
Khan MU, Pirzadeh M, Förster CY, Shityakov S, Shariati MA. Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives. Biomolecules. 2018; 8(4):110. https://doi.org/10.3390/biom8040110
Chicago/Turabian StyleKhan, Muhammad Usman, Maryam Pirzadeh, Carola Yvette Förster, Sergey Shityakov, and Mohammad Ali Shariati. 2018. "Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives" Biomolecules 8, no. 4: 110. https://doi.org/10.3390/biom8040110
APA StyleKhan, M. U., Pirzadeh, M., Förster, C. Y., Shityakov, S., & Shariati, M. A. (2018). Role of Milk-Derived Antibacterial Peptides in Modern Food Biotechnology: Their Synthesis, Applications and Future Perspectives. Biomolecules, 8(4), 110. https://doi.org/10.3390/biom8040110