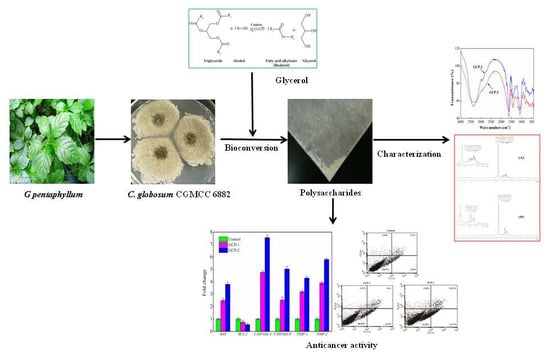
Anticancer Activity of Polysaccharides Produced from Glycerol and Crude Glycerol by an Endophytic Fungus Chaetomium globosum CGMCC 6882 on Human Lung Cancer A549 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Medium
2.1.1. Microorganism
2.1.2. Preparation of Seed Medium
2.1.3. Batch Fermentation
2.2. Characterization of GCPs Produced from Glycerol and Crude Glycerol
2.3. MTT Reduction Assay and Lactate Dehydrogenase (LDH) Activity Assay
2.4. Apoptosis Analysis by Annexin V-FITC/PI
2.5. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
3. Results and Discussion
3.1. Isolation and Purification of Polysaccharides
3.2. Characteristics of GCPs Produced from Glycerol and Crude Glycerol
3.2.1. Monosaccharide Composition and Molecular Weight of GCP-1 and GCP-2
3.2.2. FT-IR Spectroscopy of GCP-1 and GCP-2
3.2.3. NMR Analysis of GCP-1 and GCP-2
3.3. MTT and LDH Assays
3.4. Cell Apoptosis Analysis
3.5. Analysis of Gene Expression by RT-PCR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abomohra, A.E.; Eladel, H.; El-Esawi, M.; Wang, S.; Wang, Q.; He, Z.; Feng, Y.; Shang, H.; Hanelt, D. Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: Innovative waste recycling for extraordinary lipid production. Bioresour. Technol. 2018, 249, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kharmawlong, G.J.; Moholkar, V.S. Investigations in sonication-induced intensification of crude glycerol fermentation to dihydroxyacetone by free and immobilized Gluconobacter oxydans. Bioresour. Technol. 2018, 256, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Lin, J.; Luo, L.; Zhang, D.; Lei, S.; Xiao, W.; Xu, Y.; Gong, Y.; Liu, Z. Enhanced enzymatic saccharification of sugarcane bagasse pretreated by sodium methoxide with glycerol. Bioresour. Technol. 2018, 249, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Rao, C.; Xue, S.; Lei, J. Purification, characterization and neuroprotective effects of a polysaccharide from Gynostemma pentaphyllum. Carbohydr. Polym. 2015, 122, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.L.; Zhou, P.P.; Meng, X.H.; Shi, Y.P. Further new gypenosides from Jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2017, 65, 5926–5934. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Li, S.; Zhang, X.; Xu, Y.; Gao, Y.; Du, Q.; Wang, G.; Fan, W.; Sun, K.; Bian, J. Evaluation of anti-obesity activity, acute toxicity, and subacute toxicity of probiotic dark tea. Biomolecules 2018, 8, 99. [Google Scholar] [CrossRef]
- Zhang, X. Tea and cancer prevention. J. Cancer Res. Updates 2015, 4, 65–73. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Venugopalan, A.; Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015, 33, 873–887. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Pandey, S.P.; Spiteller, M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 2013, 91, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Activation of glycerol metabolism in Xanthomonas campestris by adaptive evolution to produce a high-transparency and low-viscosity xanthan gum from glycerol. Bioresour. Technol. 2016, 211, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Zhu, L.; Zhan, X. Characterization of xanthan gum produced from glycerol by a mutant strain Xanthomonas campestris CCTCC M2015714. Carbohydr. Polym. 2017, 157, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, C.; Liu, Y.; Dai, S.; Lu, W.; Lv, X.; Yao, W.; Gao, X. Preparation, characterization, and α-glycosidase inhibition activity of a carboxymethylated polysaccharide from the residue of Sarcandra glabra (Thunb.) Nakai. Int. J. Biol. Macromol. 2017, 99, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xue, X.; Zhang, Z. Structural, physicochemical, antioxidant and antitumor property of an acidic polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2017, 96, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, W.; Song, Z.; Xiong, Q.; Xu, Y.; Han, Y.; Yuan, J.; Zhang, R.; Cheng, Y.; Fang, J.; et al. Anticancer activity of polysaccharide from Glehnia littoralis on human lung cancer cell line A549. Int. J. Biol. Macromol. 2018, 106, 464–472. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, Y.; Wu, X.L.; Liu, L.Z.; Fu, W.; Song, D.D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, P.; Zhang, X.; Ravichandran, N.; Ying, H.; Yu, C.; Ying, H.; Xu, Y.; Yin, J.; Wang, K.; et al. New epigallocatechin gallate (EGCG) nanocomplexes co-assembled with 3-mercapto-1-hexanol and β-lactoglobulin for improvement of antitumor activity. J. Biomed. Nanotechnol. 2017, 13, 805–814. [Google Scholar] [CrossRef]
- Abel, S.D.A.; Baird, S.K. Honey is cytotoxic towards prostate cancer cells but interacts with the MTT reagent: Considerations for the choice of cell viability assay. Food Chem. 2018, 241, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Chun, B.S. Molecular modification of native coffee polysaccharide using subcritical water treatment: Structural characterization, antioxidant, and DNA protecting activities. Int. J. Biol. Macromol. 2017, 99, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.B.; Hua, D.; Yan, C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Ya, G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2017, 103, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, C.; Avcı, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, J.; Lang, Y.; Yao, L.; Xu, H.; Shi, H.; Xu, S. A polysaccharide from Armillaria mellea exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. Int. J. Biol. Macromol. 2012, 51, 663–667. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]






| Items | GCP-1 | GCP-2 |
|---|---|---|
| Galactose (umol/L) | 5.95 | 8.16 |
| Glucose (umol/L) | 58.75 | 43.77 |
| Mannose (umol/L) | 5.65 | 5.84 |
| Glucuronic acid (umol/L) | 0.76 | 0.43 |
| Weight-average molecular weight (Mw, Da) | 5.340 × 104 | 3.105 × 104 |
| Number-average molecular weight (Mn, Da) | 5.276 × 104 | 2.885 × 104 |
| Polydispersity (Mw/Mn) | 1.012 | 1.076 |
| Absorption Band (cm−1) | Functional Groups |
|---|---|
| 3400 | hydroxyl stretching vibration |
| 2940 | O-H stretching vibration and C-H asymmetric vibration |
| 1625 | carboxyl vibration |
| 1430 | carboxyl vibration |
| 1150 | C-C stretching vibration |
| 1000 | C-O stretching vibration |
| Absorption Signal (ppm) | Chemical Bonds |
|---|---|
| 4.7 | H in D2O |
| 4.2 | methylene in –COOC2H5 |
| 4.0 | H in hydroxyl group |
| 3.6 | H in hydroxyl group |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, P.; Tao, N.; Zhang, H.; Li, R.; Zhan, X.; Wang, F.; Shen, Y. Anticancer Activity of Polysaccharides Produced from Glycerol and Crude Glycerol by an Endophytic Fungus Chaetomium globosum CGMCC 6882 on Human Lung Cancer A549 Cells. Biomolecules 2018, 8, 171. https://doi.org/10.3390/biom8040171
Wang Z, Chen P, Tao N, Zhang H, Li R, Zhan X, Wang F, Shen Y. Anticancer Activity of Polysaccharides Produced from Glycerol and Crude Glycerol by an Endophytic Fungus Chaetomium globosum CGMCC 6882 on Human Lung Cancer A549 Cells. Biomolecules. 2018; 8(4):171. https://doi.org/10.3390/biom8040171
Chicago/Turabian StyleWang, Zichao, Peizhang Chen, Ning Tao, Huiru Zhang, Ruifang Li, Xiaobei Zhan, Fuzhuan Wang, and Yingben Shen. 2018. "Anticancer Activity of Polysaccharides Produced from Glycerol and Crude Glycerol by an Endophytic Fungus Chaetomium globosum CGMCC 6882 on Human Lung Cancer A549 Cells" Biomolecules 8, no. 4: 171. https://doi.org/10.3390/biom8040171
APA StyleWang, Z., Chen, P., Tao, N., Zhang, H., Li, R., Zhan, X., Wang, F., & Shen, Y. (2018). Anticancer Activity of Polysaccharides Produced from Glycerol and Crude Glycerol by an Endophytic Fungus Chaetomium globosum CGMCC 6882 on Human Lung Cancer A549 Cells. Biomolecules, 8(4), 171. https://doi.org/10.3390/biom8040171