Identification and Isolation of Active Compounds from Astragalus membranaceus that Improve Insulin Secretion by Regulating Pancreatic β-Cell Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Cell Culture
2.5. Cell Viability
2.6. GSIS Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Compounds 1–9
3.2. Effect of ASME and Compounds 1–9 on GSIS
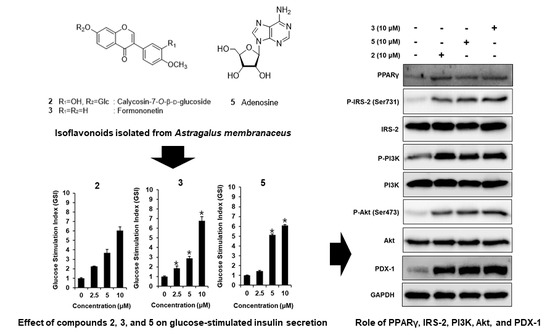
3.3. Effect of Compounds 2, 3, and 5 Isolated from A. membranaceus on the Protein Expression of PPARγ, P-IRS-2, IRS-2 (Ser731), P-PI3K, PI3K, P-Akt (Ser473), Akt, and PDX-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shrestha, P.; Ghimire, L. A Review about the Effect of Life style Modification on Diabetes and Quality of Life. Glob. J. Health Sci. 2012, 4, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65. [Google Scholar]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; A Ganai, B. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Boil. 2015, 13, 33. [Google Scholar]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 2015, 14, 57. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 28. [Google Scholar] [CrossRef]
- Liu, X.; Wei, J.; Tan, F.; Zhou, S.; Würthwein, G.; Rohdewald, P. Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004, 75, 2505–2513. [Google Scholar] [CrossRef]
- Ríos, J.; Francini, F.; Schinella, G. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Medica 2015, 81, 975–994. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network Pharmacology Studies on the Bioactive Compounds and Action Mechanisms of Natural Products for the Treatment of Diabetes Mellitus: A Review. Front. Pharmacol. 2017, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.J.; Day, C. Metformin: Its botanical background. Pract. Diabetes Int. 2004, 21, 115–117. [Google Scholar] [CrossRef]
- Group, D.P.P.R. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737. [Google Scholar]
- Agyemang, K.; Han, L.; Liu, E.; Zhang, Y.; Wang, T.; Gao, X. Recent Advances in Astragalus membranaceus Anti-Diabetic Research: Pharmacological Effects of Its Phytochemical Constituents. Evidence-Based Complement. Altern. Med. 2013, 2013, 654643. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, E.; Kwon, S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 140063. [Google Scholar] [CrossRef] [PubMed]
- Kai, Z.; Michela, P.; Antonio, P.; Annamaria, P. Biological Active Ingredients of Traditional Chinese Herb Astragalus membranaceus on Treatment of Diabetes: A Systematic Review. Mini-Rev. Med. Chem. 2015, 15, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Lavle, N.; Shukla, P.; Panchal, A. Role of flavonoids and saponins in the treatment of diabetes mellitus. J. Pharm. 2016, 6, 41–53. [Google Scholar]
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2019, 9, 122. [Google Scholar] [CrossRef]
- Gupta, D.; Kono, T.; Evans-Molina, C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes. Metab. 2010, 12, 1036–1047. [Google Scholar] [CrossRef]
- Kim, H.-S.; Hwang, Y.-C.; Koo, S.-H.; Park, K.S.; Lee, M.-S.; Kim, K.-W.; Lee, M.-K. PPAR-γ Activation Increases Insulin Secretion through the Up-regulation of the Free Fatty Acid Receptor GPR40 in Pancreatic β-Cells. PLoS ONE 2013, 8, e50128. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; Cole, B.M.; Steele, P.J. Peroxisome proliferator-activated receptor gamma (PPARγ) and its ligands: A review. Domest. Anim. Endocrinol. 2002, 22, 1–23. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; De Bittencourt, P.I.H.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxidative Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.B.; De Plata, C.A. Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb. Medica 2012, 43, 235–243. [Google Scholar]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic Activity of Sanguiin H-6 through Activation of Estrogen Receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef]
- Roy, A.; Park, H.-J.; Jung, H.A.; Choi, J.S. Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 13–20. [Google Scholar] [CrossRef]
- Chun-qing, S.; Zhi-ren, Z.; Di, L.; Zhi-bi, H. Isoflavones from Astragalus membranaceus. Acta. Bot. Sin. 1997, 39, 764–768. [Google Scholar]
- Yu, D.-H.; Bao, Y.-M.; Wei, C.-L.; An, L.-J. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 2005, 18, 297–301. [Google Scholar]
- Han, T.; Li, H.; Zhang, Q.; Zheng, H.; Qin, L. New thiazinediones and other components from Xanthium strumarium. Chem. Nat. Compd. 2006, 42, 567–570. [Google Scholar] [CrossRef]
- Choi, C.W.; Choi, Y.H.; Cha, M.-R.; Yoo, D.S.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Kim, Y.H.; Ryu, S.Y. Yeast α-Glucosidase Inhibition by Isoflavones from Plants of Leguminosae as an in Vitro Alternative to Acarbose. J. Agric. Food Chem. 2010, 58, 9988–9993. [Google Scholar] [CrossRef]
- Patching, S.G.; Baldwin, S.A.; Baldwin, A.D.; Young, J.D.; Gallagher, M.P.; Henderson, P.J.F.; Herbert, R.B. The nucleoside transport proteins, NupC and NupG, from Escherichia coli: specific structural motifs necessary for the binding of ligands. Org. Biomol. Chem. 2005, 3, 462–470. [Google Scholar] [CrossRef]
- Jiang, Y.; Choi, H.G.; Li, Y.; Park, Y.M.; Lee, J.H.; Kim, D.H.; Lee, J.-H.; Son, J.K.; Na, M.K.; Lee, S.H. Chemical constituents of Cynanchum wilfordii and the chemotaxonomy of two species of the family Asclepiadacease, C. wilfordii and C. auriculatum. Arch. Pharm. Res. 2011, 34, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Zhou, Y.; Lui, H.; Furuya, T. Astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry 1994, 36, 665–670. [Google Scholar] [CrossRef]
- Kim, J.S.; Yean, M.H.; Lee, S.Y.; Kang, S.S. Phytochemical studies on Astragalus root (1)-Saponins. Nat. Prod. Sci. 2008, 14, 14–37. [Google Scholar]
- Zou, F.; Mao, X.-Q.; Wang, N.; Liu, J.; Ou-Yang, J.-P. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol. Sin. 2009, 30, 1607–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Zhang, D.; Mao, X.; Zou, F.; Jin, H.; Ouyang, J. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol. Cell. Endocrinol. 2009, 307, 89–98. [Google Scholar] [CrossRef]
- Liao, W.; Shi, Y. Effect of astragalus polysaccharides and soy isoflavones on glucose metabolism in diabetic rats. Acta Acad. Med. Mil. Tertiae 2007, 29, 416–418. [Google Scholar]
- Ida, S.; Kaneko, R.; Murata, K. Effects of oral antidiabetic drugs on left ventricular mass in patients with type 2 diabetes mellitus: a network meta-analysis. Cardiovasc. Diabetol. 2018, 17, 129. [Google Scholar] [CrossRef]
- Van Haeften, T.; Veneman, T.; Gerich, J.; Van Der Veen, E. Influence of gliclazide on glucose-stimulated insulin release in man. Metabolism 1991, 40, 751–755. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Treatment in Type 2 Diabetic Rats Reduces Insulin Resistance and Hyperglycemia. Front. Pharmacol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPAR and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Amoroso, R. Inhibition of PPARγ by Natural Compounds as a Promising Strategy in Obesity and Diabetes. Open Med. Chem. J. 2019, 13, 7–15. [Google Scholar] [CrossRef]
- Remedi, M.S.; Emfinger, C. Pancreatic β-cell identity in diabetes. Diabetes Obes. Metab. 2016, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Pan, T.-M. A novel PPARgamma agonist monascin’s potential application in diabetes prevention. Food Funct. 2014, 5, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, S.; D’Aleo, V.; Lupi, R.; Masini, M.; Bugliani, M.; Boggi, U.; Filipponi, F.; Marchetti, P. Effects of exposure of human islet beta-cells to normal and high glucose levels with or without gliclazide or glibenclamide. Diabetes Metab. 2009, 35, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.-M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900. [Google Scholar] [CrossRef]
- Keegan, A.D.; Zamorano, J.; Keselman, A.; Heller, N.M. IL-4 and IL-13 Receptor Signaling From 4PS to Insulin Receptor Substrate 2: There and Back Again, a Historical View. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Lin, X.; Taguchi, A.; Park, S.; Kushner, J.A.; Li, F.; Li, Y.; White, M.F. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J. Clin. Investig. 2004, 114, 908–916. [Google Scholar] [CrossRef]
- Mohanty, S.; Spinas, G.; Maedler, K.; Zuellig, R.; Lehmann, R.; Donath, M.; Trüb, T.; Niessen, M. Overexpression of IRS2 in isolated pancreatic islets causes proliferation and protects human β-cells from hyperglycemia-induced apoptosis. Exp. Cell Res. 2005, 303, 68–78. [Google Scholar] [CrossRef]
- Gunton, J.E.; Kulkarni, R.N.; Yim, S.; Okada, T.; Hawthorne, W.J.; Tseng, Y.-H.; Roberson, R.S.; Ricordi, C.; O’Connell, P.J.; Gonzalez, F.J.; et al. Loss of ARNT/HIF1β Mediates Altered Gene Expression and Pancreatic-Islet Dysfunction in Human Type 2 Diabetes. Cell 2005, 122, 337–349. [Google Scholar] [CrossRef]
- Choi, M.R.; Kwak, S.M.; Bang, S.H.; Jeong, J.E.; Kim, D.J. Chronic saponin treatment attenuates damage to the pancreas in chronic alcohol-treated diabetic rats. J. Ginseng Res. 2017, 41, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.H.; Kim, J.E.; Kim, Y.S.; Ryu, C.H.; Lee, H.J.; Kim, H.M.; Jeon, H.; Won, H.-J.; Lee, J.-Y.; et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 2018, 42, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Islam, M.S. Spice-Derived Bioactive Ingredients: Potential Agents or Food Adjuvant in the Management of Diabetes Mellitus. Front. Pharmacol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, D.H.; Choi, S.; Lee, J.S.; Jang, D.S.; Kang, K.S. Identification and Isolation of Active Compounds from Astragalus membranaceus that Improve Insulin Secretion by Regulating Pancreatic β-Cell Metabolism. Biomolecules 2019, 9, 618. https://doi.org/10.3390/biom9100618
Lee D, Lee DH, Choi S, Lee JS, Jang DS, Kang KS. Identification and Isolation of Active Compounds from Astragalus membranaceus that Improve Insulin Secretion by Regulating Pancreatic β-Cell Metabolism. Biomolecules. 2019; 9(10):618. https://doi.org/10.3390/biom9100618
Chicago/Turabian StyleLee, Dahae, Da Hye Lee, Sungyoul Choi, Jin Su Lee, Dae Sik Jang, and Ki Sung Kang. 2019. "Identification and Isolation of Active Compounds from Astragalus membranaceus that Improve Insulin Secretion by Regulating Pancreatic β-Cell Metabolism" Biomolecules 9, no. 10: 618. https://doi.org/10.3390/biom9100618
APA StyleLee, D., Lee, D. H., Choi, S., Lee, J. S., Jang, D. S., & Kang, K. S. (2019). Identification and Isolation of Active Compounds from Astragalus membranaceus that Improve Insulin Secretion by Regulating Pancreatic β-Cell Metabolism. Biomolecules, 9(10), 618. https://doi.org/10.3390/biom9100618