Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health
Abstract
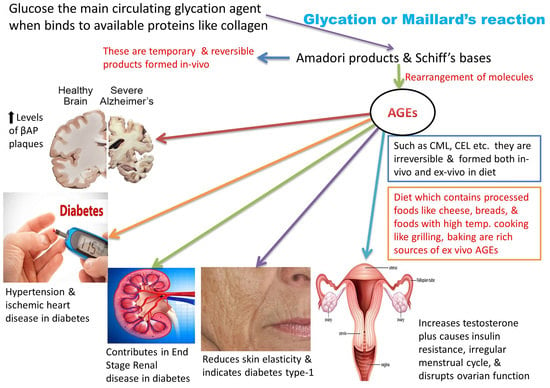
:1. Introduction: A Brief Glance at Advanced Glycation End Products (AGEs)
2. Advanced Glycation End Products (AGEs) and Modern Diet
3. AGEs: Formation and Absorption
- CML: Nε-carboxymethyl-lysine;
- CEL: Nε-1-carboxyethyl-lysine;
- Pyrraline;
- Glyoxal;
- Methylglyoxal;
- Acrylamide;
- Furan [27];
- Derivatives of bis(lysyl)imidazolium:
- DOLD: Deoxyglucosone-derived lysine dimer[1,3-di(Nε-lysino)-4(2,3,4-trihydroxybutyl)-imidazolium salt]
- GOLD: glyoxal-derived lysine dimer[1,3-di(Nε-lysino) imidazolium salt].
4. Food Processing and AGEs
5. Exposure of Infants and Toddlers to Exogenous AGEs
6. AGEs: Health and Disease
6.1. AGEs, Diabetes, and Their Related Disorders
6.2. AGEs and Brain Disorders
6.3. AGEs and Women’s Health
6.4. AGEs and Allergies
6.5. AGEs and Dental Disorder
7. Conclusions
8. Limitations
- Our review is limited to the biochemical basis of AGEs. However, serum AGEs are regulated by a receptor-mediated pathway known as the AGE-RAGE system; this mechanism gives us a broader view of their functioning. Glycation is a post-translational modification and requires an understanding of the genetic basis of this modification, efficacy of advanced glycation in the molecular basis of disease, and persistence of certain diseases. Therapeutic interventions to reduce the effects of advanced glycation in maintaining the pathophysiology of diseases, such as AD, dementia, diabetes, PCOS, ESR, aging, etc., were not covered by this review.
- Role of AGEs in oxidative stress is a limiting factor of this review
- We have described limited knowledge to understand the relationship between PCOS and AGEs. Deeper understanding can reveal newer aspects of PCOS that later manifest as infertility, thereby degrading a woman’s health.
Funding
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End Products |
| AMH | Anti-Müllerian hormone |
| PCOS | Polycystic ovary syndrome |
| AD | Alzheimer’s disease |
| DM | Diabetes mellitus |
| CML | Carboxy-methyl lysine |
| CEL | Carboxy-ethyl lysine |
References
- Steinhart, H. The Maillard Reaction. Chemistry, Biochemistry and Implications. By Harry Nursten. Angew. Int. Ed. 2005, 44, 7503–7504. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Munch, G.; Schicktanz, D.; Behme, A.; Gerlach, M.; Riederer, P.; Palm, D.; Schinzel, R. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat. Biotechnol. 1999, 17, 1006–1010. [Google Scholar] [CrossRef]
- Nowotny, K.; Schroter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Vasan, S.; Foiles, P.; Founds, H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch. Biochem. Biophys. 2003, 419, 89–96. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Nass, N.; Bartling, B.; Navarrete Santos, A.; Scheubel, R.J.; Borgermann, J.; Silber, R.E.; Simm, A. Advanced glycation end products, diabetes and ageing. Z. Gerontol. Geriatr. 2007, 40, 349–356. [Google Scholar] [CrossRef]
- Makita, Z.; Vlassara, H.; Cerami, A.; Bucala, R. Immunochemical detection of advanced glycosylation end products in vivo. J. Biol. Chem. 1992, 267, 5133–5138. [Google Scholar]
- Kasper, M.; Funk, R.H. Age-related changes in cells and tissues due to advanced glycation end products (AGEs). Arch. Gerontol. Geriatr. 2001, 32, 233–243. [Google Scholar] [CrossRef]
- Ko, S.Y.; Ko, H.A.; Chu, K.H.; Shieh, T.M.; Chi, T.C.; Chen, H.I.; Chang, W.C.; Chang, S.S. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, A.; Aschner, M. Advanced Glycation End-Products and Their Receptors: Related Pathologies, Recent Therapeutic Strategies, and a Potential Model for Future Neurodegeneration Studies. Chem. Res. Toxicol. 2016, 29, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.; Ray, R.; Banach, M.; Rai, V. Receptor for advanced glycation end-products in neurodegenerative diseases. Rev. Neurosci. 2015, 26, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine-and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [CrossRef]
- Ravichandran, G.; Lakshmanan, D.K.; Raju, K.; Elangovan, A.; Nambirajan, G.; Devanesan, A.A.; Thilagar, S. Food advanced glycation end products as potential endocrine disruptors: An emerging threat to contemporary and future generation. Environ. Int. 2019, 123, 486–500. [Google Scholar] [CrossRef]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Polycystic ovary syndrome and environmental toxins. Fertil. Steril. 2016, 106, 948–958. [Google Scholar] [CrossRef] [Green Version]
- Kutlu, T. Dietary glycotoxins and infant formulas. Turk Pediatr. 2016, 51, 179–185. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Baye, E.; de Courten, M.P.; Walker, K.; Ranasinha, S.; Earnest, A.; Forbes, J.M.; de Courten, B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: A randomised crossover trial. Sci. Rep. 2017, 7, 4123. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H.; Fuh, H.; Makita, Z.; Krungkrai, S.; Cerami, A.; Bucala, R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc. Natl. Acad. Sci. USA 1992, 89, 12043–12047. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Ojeda, A.; Jaramillo-Ortiz, S.; Wrobel, K.; Wrobel, K.; Barbosa-Sabanero, G.; Luevano-Contreras, C.; de la Maza, M.P.; Uribarri, J.; Del Castillo, M.D.; Garay-Sevilla, M.E. Comparative evaluation of three different ELISA assays and HPLC-ESI-ITMS/MS for the analysis of N(epsilon)-carboxymethyl lysine in food samples. Food Chem. 2018, 243, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wellner, A.; Huettl, C.; Henle, T. Formation of Maillard reaction products during heat treatment of carrots. J. Agric. Food Chem. 2011, 59, 7992–7998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, Q.; Jin, C.; Cheng, L.; Zheng, X.; Dai, M.; Zhang, Y. Simultaneous analysis of Nε-(carboxymethyl)Lysine and Nε-(carboxyethyl)lysine in foods by ultra-performance liquid chromatography-mass spectrometry with derivatization by 9-fluorenylmethyl chloroformate. J. Food Sci. 2015, 80, C207–C217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cocklin, R.R.; Bidasee, K.R.; Wang, M. Rapid determination of advanced glycation end products of proteins using MALDI-TOF-MS and PERL script peptide searching algorithm. J. Biomol. Tech. 2003, 14, 224–230. [Google Scholar] [PubMed]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.G.; Bailey, A.J. Glycation of collagen: The basis of its central role in the late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996, 28, 1297–1310. [Google Scholar]
- Uribarri, J. Dietary AGE and Their Role in Health and Disease; CRCPress: BocaRaton, FL, USA, 2018. [Google Scholar]
- Koschinsky, T.; He, C.-J.; Mitsuhashi, T.; Bucala, R.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [Green Version]
- Snelson, M.; Coughlan M., T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of age formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Grunwald, S.; Krause, R.; Bruch, M.; Henle, T.; Brandsch, M. Transepithelial flux of early and advanced glycation compounds across caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. Br. J. Nutr. 2006, 95, 1221–1228. [Google Scholar] [CrossRef]
- Hellwig, M.; Matthes, R.; Peto, A.; Lobner, J.; Henle, T. N-epsilon-fructosyllysine and n-epsilon-carboxymethyllysine, but not lysinoalanine, are available for absorption after simulated gastrointestinal digestion. Amino Acids. 2014, 46, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Teodorowicz, M.; Neerven, J.V.; Savelkoul, H. Food Processing: The Influence of the Maillard Reaction on Immunogenicity and Allergenicity of Food Proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.; Helling, R.; Heichert, C.; Scheunemann, M.; Mäding, P.; Wittrisch, H.; Johannsen, B.; Henle, T. Radio fluorination and positron emission tomography (PET) as a new approach to study the in vivo distribution and elimination of the advanced glycation endproducts N-epsilon-carboxymethyllysine (CML) and N-epsilon-carboxyethyllysine (CEL)and N epsilon-carboxyethyllysine (CEL). Nahrung 2001, 45, 182–188. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Guilbaud, A.; Niquet-Leridon, C.; Boulanger, E.; Tessier, F.J. How Can Diet Affect the Accumulation of Advanced Glycation End-Products in the Human Body? Foods 2016, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macias-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Henle, T. Dietary advanced glycation end products—A risk to human health? A call for an interdisciplinary debate. Mol. Nutr. Food Res. 2007, 51, 1075–1078. [Google Scholar] [CrossRef]
- Cai, W.; Uribarri, J.; Zhu, L.; Chen, X.; Swamy, S.; Zhao, Z.; Grosjean, F.; Simonaro, C.; Kuchel, G.A.; Schnaider-Beeri, M.; et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA 2014, 111, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Imrhan, V. Advanced Glycation End Products, Inflammation, and Chronic Metabolic Diseases: Links in a Chain? Crit. Rev. Food Sci. Nutr. 2016, 56, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 2016, 32, 157–165. [Google Scholar] [CrossRef]
- Illien-Junger, S.; Lu, Y.; Qureshi, S.A.; Hecht, A.C.; Cai, W.; Vlassara, H.; Striker, G.E.; Iatridis, J.C. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PLoS ONE 2015, 10, e0116625. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, P.M.; Appell, H.J.; Duarte, J.A. Involvement of advanced glycation end products in the pathogenesis of diabetic complications: The protective role of regular physical activity. Eur. Rev. Aging Phys. Act. 2008, 5, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.Y.; Li, S.M.; Wang, Y.; Tan, D.; Pan, M.H.; Sang, S.M.; Ho, C.T. Reactive dicarbonyl compounds and 5-(hydroxymethyl)-2-furfural in carbonated beverages containing high fructose corn syrup. Food Chem. 2008, 107, 1099–1105. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- EFSA (European food safety authority). Update on acrylamide levels in food from monitoring years 2007–2010. EFSA J. 2012, 10, 2938–2976. [Google Scholar] [CrossRef]
- Mariotti, M.; Toledo, C.; Hevia, K.; Gómez, J.P.; Fromberg, A.; Granby, K.; Rosowski, J.; Castillo, O.; Pedreschi, F. Are Chileans exposed to dietary furan? Food Addit. Contam. Part A 2013, 10, 1715–1721. [Google Scholar]
- Aguirre, D.B.; Corradini, M.G.; Candogan, K.; Barbosa-Canovas, G.V. High pressure processing in combination with high temperature and other preservation factors. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 193–215. [Google Scholar] [CrossRef]
- Albala-Hurtado, S.; Veciana-Nogues, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Determination of free and total furfural compounds in infant milk formulas by high-performance liquid chromatography. J. Agric. Food Chem. 1997, 45, 2128–2133. [Google Scholar] [CrossRef]
- Prosser, C.G.; Carpenter, E.A.; Hodgkinson, A.J. N(epsilon)-carboxymethyllysine in nutritional milk formulas for infants. Food Chem. 2019, 274, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Gleeson, H.K. Long-term and late treatment consequences: Endocrine and metabolic effects. Curr. Opin. Support Palliat. Care 2017, 11, 205–213. [Google Scholar] [CrossRef]
- Gupta, A.; Uribarri, J. Dietary Advanced Glycation End Products and Their Potential Role in Cardiometabolic Disease in Children. Horm. Res. Paediatr. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.W.; Wu, M.H.; Tu, L.H. Protein Glycation by glyoxal promotes amyloid formation by islets amyloid polypeptide. Biophys. J. 2019, 116, 2304–2313. [Google Scholar] [CrossRef]
- Hanyu, H. Diabetes –related Dementia. Diabetes Mellit. 2019, 1128, 147–160. [Google Scholar] [CrossRef]
- Chou, P.S.; Wu, M.N.; Yang, C.C.; Shen, C.T.; Yang, Y.H. Effect of advanced glycation end products on the progression of Alzheimer’s disease. J. Alzheimers Dis. 2019. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Amad, A.; Arai, M.; Miyashita, M.; Lalau, J.D.; Loas, G.; Itokawa, M. Advanced glycation end products and schizophrenia: A systematic review. J Psychiatr. Res. 2015, 66, 112–117. [Google Scholar] [CrossRef]
- Clatici, V.G.; Voicu, C.; Kalashnicova, N.G.; Fica, S. Glycation: Implication in perceived age and dermatology. Rom. J. Clin. Experi. Dermatol. 2017, 4, 114–123. [Google Scholar] [CrossRef]
- Kirstein, M.; Brett, J.; Radoff, S.; Ogawa, S.; Stern, D.; Vlassara, H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: Role in vascular disease of diabetes and aging. Proc. Natl. Acad. Sci. USA 1990, 87, 9010–9014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehrsson, S.K.; Jonasson, R.; Lins, L.E. Cardiac performance in various stages of renal failure. Br. Heart J. 1984, 52, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyurászová, M.; Kovalčíková, A.G.; Renczés, E.; Kmeťová, K.; Celec, P.; Bábíčková, J.; Tóthová, L. Oxidative Stress in Animal Models of Acute and Chronic Renal Failure. Dis. Markers 2019. [Google Scholar] [CrossRef] [Green Version]
- De Gooyer, T.E.; Stevenson, K.A.; Humphries, P.; Simpson, D.A.; Gardiner, T.A.; Stitt, A.W. Retinopathy Is Reduced during Experimental Diabetes in a Mouse Model of Outer Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5561–5568. [Google Scholar] [CrossRef] [Green Version]
- Uchiki, T.; Weikel, K.A.; Jiao, W.; Shang, F.; Caceres, A.; Pawlak, D.; Handa, J.T.; Brownlee, M.; Nagaraj, R.; Taylor, A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics). Aging Cell 2012, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hegab, Z.; Gobbons, S.; Neyses, L.; Mamas, A.M. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Derm. Endocrinol. 2012, 3, 259–270 doiorg/104161/derm22028. [Google Scholar] [CrossRef] [Green Version]
- Sell, D.R.; Monnier, V.M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J. Clin. Investig. 1990, 85, 380–384. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Kandaraki, E.; Piperi, C.; Livadas, S.; Papavassiliou, A.G.; Koutsilieris, M.; Papalois, A.; Diamanti-Kandarakis, E. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J. Endocrinol. 2013, 218, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Mondal, L.K.; Gautam Bhaduri, G.; Bhattacharya, B. Biochemical scenario behind initiation of diabetic retinopathy in type 2 diabetes mellitus. Indian J. Ophthalmol. 2018, 66, 535–540. [Google Scholar] [CrossRef]
- Bucala, R.; Makita, Z.; Vega, G.; Grundy, S.; Koschinsky, T.; Cerami, A.; Vlassara, H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc. Natl. Acad. Sci. USA 1994, 91, 9441–9445. [Google Scholar]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.K. Do advanced glycation end-products cause food allergy? Curr. Opin. Allergy Clin. Immunol. 2017, 17, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Soldatos, G.; Thomas, M.C. Below the radar: Advanced glycation end products that detour “around the side”. Is HbA1c not an accurate enough predictor of long term progression and glycaemic control in diabetes? Clin. Biochem. Rev. 2005, 26, 123–134. [Google Scholar]
- Dozio, E.; Corradi, V.; Vianello, E.; Scalzotto, E.; de Cal, M.; Corsi Romanelli, M.M.; Ronco, C. Increased Levels of sRAGE in Diabetic CKD-G5D Patients: A Potential Protective Mechanism against AGE-Related Upregulation of Fibroblast Growth Factor 23 and Inflammation. Mediat. Inflamm. 2017, 2017, 9845175. [Google Scholar] [CrossRef] [Green Version]
- Kanda, A.; Dong, Y.; Noda, K.; Saito, W.; Ishida, S. Advanced glycation endproducts link inflammatory cues to upregulation of galectin-1 in diabetic retinopathy. Sci. Rep. 2017, 7, 16168. [Google Scholar] [CrossRef]
- Wada, R.; Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005, 1043, 598–604. [Google Scholar] [CrossRef]
- Normand, G.; Lemoine, S.; Villien, M.; Le Bars, D.; Merida, I.; Irace, Z.; Troalen, T.; Costes, N.; Juillard, L. AGE Content of a Protein Load Is Responsible for Renal Performances: A Pilot Study. Diabetes Care 2018, 41, 1292–1294. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, S.; Matsui, T.; Takeuchi, M.; Yamagishi, S.I. Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis. Horm. Metab. Res. 2014, 46, 717–721. [Google Scholar] [CrossRef]
- Nakamura, Y.; Horii, Y.; Nishino, T.; Shiiki, H.; Sakaguchi, Y.; Kagoshima, T.; Dohi, K.; Makita, Z.; Vlassara, H.; Bucala, R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 1993, 143, 1649–1656. [Google Scholar]
- Manicam, C.; Ginter, N.; Li, H.; Xia, N.; Goloborodko, E.; Zadeh, J.K.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Compensatory Vasodilator Mechanisms in the Ophthalmic Artery of Endothelial Nitric Oxide Synthase Gene Knockout Mice. Sci. Rep. 2017, 7, 7111. [Google Scholar] [CrossRef] [Green Version]
- Bucala, R.; Makita, Z.; Koschinsky, T.; Cerami, A.; Vlassara, H. Lipid advanced glycosylation: Pathway for lipid oxidation in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 6434–6438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teissier, T.; Quersin, V.; Gnemmi, V.; Daroux, M.; Howsam, M.; Delguste, F.; Lemoine, C.; Fradin, C.; Schmidt, A.M.; Cauffiez, C.; et al. Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions. Aging Cell 2019, 18, e12850. [Google Scholar] [CrossRef] [PubMed]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Cerami, A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 4766–4770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Do Advanced Glycation End Products (AGEs) Contribute to the Comorbidities of Polycystic Ovary Syndrome (PCOS)? Curr. Pharm. Des. 2016, 22, 5558–5571. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Kandaraki, E.; Piouka, A.; Papavassiliou, A.G.; Panidis, D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Merhi, Z. Relationship between Advanced Glycation End Products and Steroidogenesis in PCOS. Reprod. Biol. Endocrinol. 2016, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Tantalaki, E.; Piperi, C.; Livadas, S.; Kollias, A.; Adamopoulos, C.; Koulouri, A.; Christakou, C.; Diamanti-Kandarakis, E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones 2014, 13, 65–73. [Google Scholar] [CrossRef]
- Merhi, Z.; Kandaraki, E.A.; Diamanti-Kandarakis, E. Implications and Future Perspectives of AGEs in PCOS Pathophysiology. Trends Endocrinol. Metab. 2019, 30, 150–162. [Google Scholar] [CrossRef]
- Lin, P.H.; Chang, C.C.; Wu, K.H.; Shih, C.K.; Chiang, W.; Chen, H.Y.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M.; et al. Dietary Glycotoxins, Advanced Glycation End Products, Inhibit Cell Proliferation and Progesterone Secretion in Ovarian Granulosa Cells and Mimic PCOS-like Symptoms. Biomolecules 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Huang, R.; Sun, Y.; Yue, J.; Zheng, J.; Wang, L.; Tao, T.; Ma, J.; Li, S.; Liu, W. An inverse association between serum soluble receptor of advanced glycation end products and hyperandrogenism and potential implication in polycystic ovary syndrome patients. Reprod. Biol. Endocrinol. 2017, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Diamanti-Kandarakis, E.; Lambrinoudaki, I.; Economou, F.; Christou, M.; Piperi, C.; Papavassiliou, A.; Creatsas, G. Androgens associated with advanced glycation end-products in postmenopausal women. Menopause 2010, 17, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.F. Clinical features, hormonal profile, and metabolic abnormalities of obese women with obese polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi 2005, 85, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, M.; Ciotta, L.; Palumbo, M.A. Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol. Endocrinol. 2018, 34, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Papachroni, K.K.; Piperi, C.; Levidou, G.; Korkolopoulou, P.; Pawelczyk, L.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J. Cell. Mol. Med. 2010, 14, 2460–2469. [Google Scholar] [CrossRef] [Green Version]
- Graff, S.K.; Mario, F.M.; Alves, B.C.; Spritzer, P.M. Dietary glycemic index is associated with less favorable anthropometric and metabolic profiles in polycystic ovary syndrome women with different phenotypes. Fertil. Sterl. 2013, 100, 1081–1088. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implications in reproduction: A systematic review. Fertil. Sterl. 2014, 102, 460–468. [Google Scholar] [CrossRef]
- Masjedi, F.; Keshtgar, S.; Zal, F.; Talaei-Khozani, T.; Sameti, S.; Fallahi, S.; Kazeroni, M. Effects of vitamin D on steoidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulose cells of normal and polycystic ovaries. J. Steroid Biochem. Mol. Biol. 2019, 197, 105521. [Google Scholar] [CrossRef]
- Endo, T.; Kiya, T.; Goto, T.; Henmi, H.; Manase, K.; Honnma, H.; Baba, T.; Ishioka, S.; Hayashi, T.; Arima, K.; et al. Significance of matrix metalloproteinases in pathophysiology of the ovary and uterus. Reprod. Med. Biol. 2006, 5, 235–243. [Google Scholar] [CrossRef]
- Merhi, Z. Vitamin D attenuates the effect of advanced glycation end products on anti-Mullerian hormone signaling. Mol. Cell. Endocrinol. 2019, 479, 87–92. [Google Scholar] [CrossRef]
- Henmi, H.; Endo, T.; Nagasawa, K.; Hayashi, T.; Chida, M.; Akutagawa, N.; Iwasaki, M.; Kitajima, Y.; Kiya, T.; Nishikawa, A.; et al. Lysyl oxidase and MMP-2 expression in dehydorepiandrosterone-induces polycystic ovary in rats. Biology of Reproduction. 2001, 64, 157–162. [Google Scholar] [CrossRef]
- Christakou, C.; Diamanti-Kandarakis, E. Polycystic ovary syndrome-phenotype and diagnosis. Scandinavian journal of clinical and laboratory investigation. Supplementum 2014, 244, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Livadas, S.; Diamanti-Kandarakis, E. Polycystic ovary syndrome: Definitions, phenotypes and diagnostic approach. Front. Horm. Res. 2013, 40, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Mladenovic, V.; Bjekic-Macut, J.; Livadas, S.; Stanojlovic, O.; Hrncic, D.; Rasic-Markovic, A.; Milutinovic, D.V.; Andric, Z. Hypertension in polycystic ovary syndrome: Novel insights. Curr. Hypertens. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Buyuk, E.; Cipolla, M.J. Advanced glycation end products alter steroidogenic gene expression by granulosa cells: An effect partially reversible by vitamin D. Mol. Hum. Reprod. 2018, 24, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Piperi, C.; Alexandraki, K.; Katsilambros, N.; Kouroupi, E.; Papailiou, J.; Lazaridis, S.; Koulouri, E.; Kandarakis, H.A.; Douzinas, E.E.; et al. Short-term effect of orlistat on dietary glycotoxins in healthy women and women with polycystic ovary syndrome. Metab. Clin. Exp. 2006, 55, 494–500. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Korkolopoulou, P.; Kandaraki, E.; Levidou, G.; Papalois, A.; Patsouris, E.; Papavassiliou, A.G. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J. Mol. Med. 2007, 85, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merhi, Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; Ten Boekel, E.; Wee, M.M.T.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): A cross-sectional study. PLoS ONE 2018, 13, e0204748. [Google Scholar] [CrossRef]
- Lin, M.-W.; Wu, M.-H. The role of vitamin D in polycystic ovary syndrome. Indian J. Med. Res. 2015, 142, 238–240. [Google Scholar] [CrossRef]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Nagai, R.; Burgdorf, S.; Scheurer, S.; Vieths, S.; et al. Ovalbumin modified with pyrraline, a Maillard reaction product, shows enhanced T-cell immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Alqahtani, F.; Alqahtani, M.; Al-Kheraif, A.A.; Javed, F. Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type-2 diabetes mellitus. J. Periodontol. 2019. [Google Scholar] [CrossRef] [PubMed]






| Maillard Reaction Product | Type of Foods | Food Processing | Advanced Glycation End Products Range in Different Foods | References |
|---|---|---|---|---|
| Acrylamide (a by-product of MRP) [27] | Starchy, potato-based foods, such as french fries. | Frying Grilling Baking | Fried potatoes: 272–570 µg/kg−1 Bakery products: 75–1044 µg/kg−1 Breakfast cereals: 149 µg/kg−1 | [50] |
| Furan [27] | PUFA-rich foods, carotenoids, or vitamin-containing foods. | Roasting Frying Caramelizing Pasteurization | Espresso coffee: 936 ng/g−1 Potato chips: 259 ng/g−1 Jarred baby foods: 8.5 ng/g−1 Orange juice: 7.0 ng/g−1 | [51] |
| CML [4] | Infant formula, milk and dairy products, boiled eggs, peanut butter, beef, chicken, meat. | Roasting Charring Boiling Baking Grilling Toasting | Peanut butter, chocolate sprinklers: 5–7 mg/100 g protein Milk chocolates: 0.01 mg/100 g protein Milk samples: 2.7 mg/100 g protein White bread, boiled eggs: 11.2 mg/100 g protein Grilled chicken: 5–500 µ/100 g product | [4,24] |
| CEL [4] | Sponge cakes, potato chips, peanut butter. | Baking Roasting Frying | Deep-sea fish: 2.49–249 ng/mL Peanut butter: 7 mg/100 g product | [4] |
| Methyl glyoxal, glyoxal, 3-deoxyglucosone [4] | High-fructose corn syrup | Pasteurization Boiling Baking High-pressure processing | Fruit juices: 410 mg/L Balsamic vinegar: 2622 mg/L Cookies: 385 mg/kg Carbonated soft drinks: 0.3–1 mg/L | [48,49,52] |
| Organ/Disease | Binds to | Effects |
|---|---|---|
| Brain | Amyloid protein | Increases β-amyloid plaques [58], resulting in dementia [59,60] or severity in schizophrenia [61] |
| Skin | Articular collagen, skeletal and smooth vascular muscles, glomerular basement membrane | Reduces flexibility, alterations of co-functions, such as migration, differentiation, and proliferation [62,63] |
| Kidney | Bowman’s capsule | Accumulation of uremic toxins [64], the appearance of complications, such as poly-nephropathy [8], chronic renal failure [65] |
| Eyes | Opsin | Macular degeneration of the retina [66,67] |
| Heart | Vessels | Progression of coronary heart disease or myocardial damage [68] |
| Photoaging | Fibroblasts/keratinocytes Superoxide dismutase | Cells become more sensitive to exposure to UVA radiations and their viability decreases, impairing repair mechanism [62,69]. Compromise cellular antioxidant defense system [69] |
| Joints, lungs, heart, skin, blood or combination of these, Systemic Lupus Erythematosus | White blood cells | Inflammation in mentioned organs attacking own cells, face rashes, flare, sensitivity to light, swelling, etc. [7,69,70,71] |
| Diabetes | Low-density lipoprotein | During chronic hyperglycemia, promotes the initiation of lipid peroxidation in vivo [3,62,72] Macro and microvascular complications of diabetes [73,74,75,76,77] |
| Biochemical Changes | Effects | Ref. |
|---|---|---|
| High levels of testosterone and androstenedione | Irregular menstrual cycles | [94,95,96] |
| Raised expression of Receptor of AGE (RAGE) in mononuclear cells along with increased glucose, insulin, and testosterone | PCOS characterized as both endocrine metabolic disorders | [71,95,97] |
| High AGE diet elevates anti-Müllerian hormone, inhibits Follicle-stimulating hormone | Provokes anovulation | [98,99] |
| High AGEs isocaloric diet elevates testosterone, insulin, and oxidative stress contributing to PCOS and its symptoms | Irregular menstrual cycles, high ovarian cysts | [92,97,100] |
| Upregulation of RAGE in PCOS, downregulation signal cascade of steroidogenesis in women of reproductive age | Disruptive hormone formation | [91,101] |
| Vitamin D3 supplementations reduce the effects of AGEs in PCOS | Attenuates AGEs and supports ovarian health | [102,103] |
| Excess deposition of collagen | Cyst formation in ovaries due to enzyme lysyl oxidase | [102,104,105] |
| Disruption of renin-angiotensin-aldosterone system | Disturbed cardiovascular functioning and hypertension | [37,106,107] |
| Disease | Effects of AGEs | References |
|---|---|---|
| Diabetes | Crosslinking of skin collagen, carotid thickening, ischemic heart attack, chronic and end-stage renal disease, diabetic retinopathy, uremic cardiomyopathy, alterations in lipo- and apolipoproteins, inactivation of nitric oxide | [64,70,71,72,73,74,75,76,77,78,79,80,81,82,83] |
| Alzheimer’s disease, certain neurodegenerative diseases, advance stages of amyloidosis | β-amyloid protein plaques, cerebrovascular amyloid deposits, neurofibrillary tangles | [85,86,87] |
| Ovarian dysfunction, polycystic ovarian syndrome, anovulation, infertility | Increased testosterone, thyroid hormones, androgens, anti-Mullerian hormone, disruptive steroidogenesis | [71,92,108] |
| Inflammation, allergies, asthma, multiple sclerosis, Crohn’s disease | Activating unprogrammed cell death, altering immune responses by monocytes, basophils, macrophages, and dendritic cells, might create false allergic responses | [75,76,114,115] |
| Dental disorders | Periodontitis leading to tooth loss due to gum infection might increase the risk of heart and lung diseases | [116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. https://doi.org/10.3390/biom9120888
Gill V, Kumar V, Singh K, Kumar A, Kim J-J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules. 2019; 9(12):888. https://doi.org/10.3390/biom9120888
Chicago/Turabian StyleGill, Vidhu, Vijay Kumar, Kritanjali Singh, Ashok Kumar, and Jong-Joo Kim. 2019. "Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health" Biomolecules 9, no. 12: 888. https://doi.org/10.3390/biom9120888
APA StyleGill, V., Kumar, V., Singh, K., Kumar, A., & Kim, J. -J. (2019). Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules, 9(12), 888. https://doi.org/10.3390/biom9120888