Reproductive Output and Insect Behavior in Hybrids and Apomicts from Limonium ovalifolium and L. binervosum Complexes (Plumbaginaceae) in an Open Cross-Pollination Experiment
Abstract
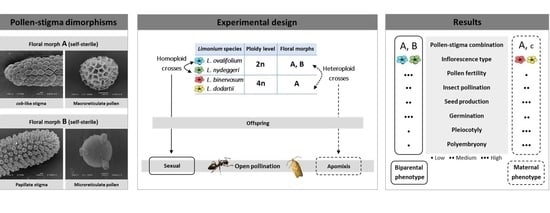
:1. Introduction
2. Results
2.1. Inflorescence Phenotype, Pollen-Stigma Combinations, and Pollen Viability
2.2. Floral Visitors
2.3. Seed Germination, Seedling Morphotype, and Ploidy Analyses
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Inflorescence Phenotype, Floral Heteromorphisms, and Pollen Viability
4.3. Open Pollination Experiment and Floral Visitors
4.4. Seed Germination and Seedlings’ Phenotype Evaluation
4.5. Genome Size and DNA Ploidy Estimations in Seedlings
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, H.G. The Evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution 1966, 20, 349–368. [Google Scholar] [CrossRef]
- Erben, M. Die Gattung Limonium im südwestmediterranen Raum. Mitt. Bot. Staatssamml. Miinchen 1978, 14, 361–631. [Google Scholar]
- Costa, J.; Castro, S.; Loureiro, J.; Barrett, S.C. Experimental insights on the function of ancillary pollen and stigma polymorphisms in plants with heteromorphic incompatibility. Evolution 2017, 71, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Conceição, S.I.R.; Róis, A.S.; Caperta, A.D. Limonium homoploid and heteroploid intra-and interspecific crosses unveil seed anomalies and neopolyploidy related to sexual and/or apomictic reproduction. Taxon 2018, 67, 1153–1162. [Google Scholar] [CrossRef]
- Dulberger, R. Intermorph structural differences between stigmatic papillae and pollen grains in relation to incompatibility in Plumbaginaceae. Proc. R. Soc. Lond. B 1975, 188, 257–274. [Google Scholar] [CrossRef]
- Dulberger, R. S-gene action and the significance of characters in the heterostylous syndrome. Heredity 1975, 35, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Róis, A.S.; Sádio, F.; Paulo, O.S.; Teixeira, G.; Paes, A.P.; Espírito-Santo, D.; Sharbel, T.F.; Caperta, A.D. Phylogeography and modes of reproduction in diploid and tetraploid halophytes of Limonium species (Plumbaginaceae): Evidence for a pattern of geographical parthenogenesis. Ann. Bot. 2016, 117, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Torices, R.; Barrett, S.C. Evolutionary history of the buildup and breakdown of the heterostylous syndrome in Plumbaginaceae. New Phytol. 2019, 224, 1278–1289. [Google Scholar] [CrossRef]
- Baker, H.G. Dimorphism and monomorphism in the Plumbaginaceae. II. Pollen and stigmata in the genus Limonium. Ann. Bot. 1953, 17, 433–445. [Google Scholar] [CrossRef]
- Baker, H.G. Corolla size in gynodioecious and gynomonoecious species of flowering plants. Proc. Leeds Phil. Soc. 1948, 5, 136–139. [Google Scholar]
- Cortinhas, A.; Erben, M.; Paes, A.P.; Santo, D.E.; Guara-Requena, M.; Caperta, A.D. Taxonomic complexity in the halophyte Limonium vulgare and related taxa (Plumbaginaceae): Insights from analysis of morphological, reproductive and karyological data. Ann. Bot. 2015, 115, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Róis, A.S.; Teixeira, G.; Sharbel, T.F.; Fuchs, J.; Martins, S.; Espírito-Santo, D.; Caperta, A.D. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders (Limonium sp., Plumbaginaceae) reveal diversity in reproduction modes. Sex. Plant. Reprod. 2012, 25, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Erben, M. Karyotype differentiation and its consequences in Mediterranean Limonium. Webbia 1979, 34, 409–417. [Google Scholar] [CrossRef]
- Boorman, L.A. Limonium vulgare Miller and L. humile Miller, in the biological flora of the British Isles. J. Ecol. 1967, 55, 221–232. [Google Scholar] [CrossRef]
- Khan, Z.; Santpere, G.; Traveset, A. Breeding system and ecological traits of the critically endangered endemic plant Limonium barceloi (Gil and Llorens) (Plumbaginaceae). Plant. Syst. Evol. 2012, 298, 1101–1110. [Google Scholar] [CrossRef]
- Koch, M.A.; Dobeš, C.; Mitchell-Olds, T. Multiple hybrid formation in natural populations: Concerted evolution of the internal transcribed spacer of nuclear ribosomal DNA (ITS) in North American Arabis divaricarpa (Brassicaceae). Mol. Bio. Evol. 2003, 20, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Schranz, M.E.; Dobeš, C.; Koch, M.A.; Mitchell-Olds, T. Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae). Amer. J. Bot. 2005, 92, 1797–1810. [Google Scholar] [CrossRef]
- Paun, O.; Greilhuber, J.; Temsch, E.M.; Hörandl, E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae). Molec. Ecol. 2006, 15, 897–910. [Google Scholar] [CrossRef]
- Kantama, L.; Sharbel, T.F.; Schranz, M.E.; Mitchell-Olds, T.; De Vries, S.; De Jong, H. Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proc. Natl. Acad. Sci. USA 2007, 104, 14026–14031. [Google Scholar] [CrossRef] [Green Version]
- Hodač, L.; Scheben, A.P.; Hojsgaard, D.; Paun, O.; Hörandl, E. ITS polymorphisms shed light on hybrid evolution in apomictic plants: A case study on the Ranunculus auricomus complex. PLoS ONE 2014, 9, e103003. [Google Scholar] [CrossRef]
- Dahlgren, K.V.O. Zytologische und embryologische Studien über die Reihen Primulales und Plumbaginales. Kungl. Sven. Vetensk. Handl. 1916, 56, 1–80. [Google Scholar]
- D’Amato, F. Triploidia e apomissia in Statice oleaefolia Scop. var. Confusa Godr. Caryologia 1949, 2, 71–84. [Google Scholar] [CrossRef]
- Hjelmqvist, H.; Grazi, F. Studies on variation in embryo sac development. Bot. Not. 1964, 117, 141–166. [Google Scholar]
- Caperta, A.D.; Castro, S.; Loureiro, J.; Róis, A.S.; Conceição, S.; Costa, J.; Rhazi, L.; Espírito Santo, D.; Arsénio, P. Biogeographical, ecological and ploidy variation in related asexual and sexual Limonium taxa (Plumbaginaceae). Bot. J. Linn. Soc. 2017, 183, 75–93. [Google Scholar] [CrossRef]
- Caperta, A.D.; Conceição, S.I.R.; Róis, A.S.; Loureiro, J.; Castro, S. Cytogenetic features of sexual and asexual Limonium taxa (Plumbaginaceae). Taxon 2018, 67, 1143–1152. [Google Scholar] [CrossRef]
- Flora Ibérica. Available online: http://www.floraiberica.es/floraiberica/texto/pdfs/03_055_04_Limonium.pdf (accessed on 12 February 2020).
- Erben, M. Limonium nydeggeri eine neue Art aus Sudwestportugal. Sendtnera 1999, 6, 103–107. [Google Scholar]
- Stace, C. New Flora of the British Isles, 3rd ed.; Cambridge University Press: Cambridge, UK, 2010; pp. 243–248. [Google Scholar]
- Róis, A.S.; López, C.M.R.; Cortinhas, A.L.; Erben, M.; Espírito-Santo, D.; Wilkinson, M.J.; Caperta, A.D. Epigenetic rather than genetic factors may explain phenotypic divergence between coastal populations of diploid and tetraploid Limonium spp. (Plumbaginaceae) in Portugal. BMC Plant. Biol. 2013, 13, 205. [Google Scholar] [CrossRef] [Green Version]
- Dobeš, C.; Scheffknecht, S.; Fenko, Y.; Prohaska, D.; Sykora, C.; Hülber, K. Asymmetric reproductive interference: The consequences of cross-pollination on reproductive success in sexual-apomictic populations of Potentilla puberula (Rosaceae). Ecol. Evol. 2018, 8, 365–381. [Google Scholar] [CrossRef] [Green Version]
- Dobeš, C.; Milosevic, A.; Prohaska, D.; Scheffknecht, S.; Sharbel, T.F.; Hülber, K. Reproductive differentiation into sexual and apomictic polyploid cytotypes in Potentilla puberula (Potentilleae, Rosaceae). Ann. Bot. 2013, 112, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Hörandl, E. Evolutionary implications of self-compatibility and reproductive fitness in the apomictic Ranunculus auricomus polyploid complex (Ranunculaceae). Int. J. Plant. Sci. 2008, 169, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Mráz, P. Mentor effects in the genus Hieracium s. str. (Compositae, Lactuceae). Folia Geob. 2003, 38, 345–350. [Google Scholar]
- Conceição, S.I.R.; Róis, A.S.; Caperta, A.D. Nonreduction via meiotic restitution and pollen heterogeneity may explain residual male fertility in triploid marine halophyte Limonium algarvense (Plumbaginaceae). Caryologia 2019, 72, 53–62. [Google Scholar] [CrossRef]
- Maia, F.R.; Varassin, I.G.; Goldenberg, R. Apomixis does not affect visitation to flowers of Melastomataceae, but pollen sterility does. Plant. Biol. 2016, 18, 132–138. [Google Scholar] [CrossRef]
- Natural History Museum. Available online: http://www.nhm.ac.uk/hosts (accessed on 25 June 2020).
- Zhang, A.Q.; He, S.; Zhai, Y.X.; Huang, S.Q. Does persistence of showy calyces in Limonium leptolobum enhance pollinator attraction? J. Plant. Ecol. 2015, 8, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Nowicke, J.W.; Skvarla, J.J. Pollen morphology and the relationship of the Plumbaginaceae, Polygonaceae, and Primulaceae to the order Centrospermae. Smithson. Contrib. Bot. 1976, 37, 1–64. [Google Scholar] [CrossRef]
- Corley, M.F.V.; Ferreira, S.; Grundy, D.; Nunes, J.; Pires, P.; Rosete, J. New and interesting Portuguese Lepidoptera records from 2017 (Insecta: Lepidoptera). SHILAP Rev. Lepidopterol. 2018, 46, 551–576. [Google Scholar]
- Clifton, J. Clepsis coriacana (Rebel, 1894) new to Europe plus further records of Clepsis peritana (Clemens, 1860) from Gibraltar (Lepidoptera: Tortricidae). SHILAP Rev. Lepidopterol. 2007, 35, 47–48. [Google Scholar]
- Wvan, C. Syncotyly, pseudomonotyly, schizocotyly and pleicotyly within some dicotyledons. Biol. Jarl. Dodonaea 1981, 49, 166–183. [Google Scholar]
- Carman, J.G. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linn. Soc. 1997, 61, 51–94. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and non-aborted pollen. Stain Tech. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Collingwood, C.; Prince, A. A guide to ants of Continental Portugal (Hymenoptera, Formicidae). Bol. Soc. Port. Entomol. 1998, 5, 49. [Google Scholar]
- On-line Systematic Catalog of Plant Bugs (Insecta: Heteroptera: Miridae). Available online: http://research.amnh.org/pbi/catalog/ (accessed on 25 June 2020).
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Doležel, J.; Greilhuber, J.; Lucretti, S.; Meister, A.; Lysák, M.A.; Nardi, L.; Obermayer, R. Plant genome size estimation by flow cytometry: Inter-laboratory comparison. Ann. Bot. 1998, 82, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Greilhuber, J.; Doležel, J.; Lysak, M.A.; Bennett, M.D. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann. Bot. 2005, 95, 255–260. [Google Scholar] [CrossRef]




| Crosses | Parental Plants | Pollen-Stigma Combinations * | Inflorescence Morphotypes | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | L. nydeggeri | Intermediate | L. ovalifolium | L. binervosum | L. dodartii | |||
| Homoploid | A♀ × B♂/B♀ × A♂ | 19 | 20 | 0 | 8 | 9 | 18 | 1 | 0 | 36 |
| Heteroploid | A♀ × B♂ | 14 | 0 | 1 | 0 | 0 | 0 | 14 | 1 | 15 |
| Trait | Type of Reproduction | Probability | LRT Statistic Value (p-Value) |
|---|---|---|---|
| Colpi number | Sexual | 0.99 | 580.85 (<2.2 × 10−16) |
| Apomixis | 0.34 | ||
| Cotyledons number | Sexual | 0.97 | 45.57 (1.477 × 10−11) |
| Apomixis | 0.81 | ||
| Pollen-stigma combination | Sexual | 0.59 | 16.61 (4.59 × 10−5) |
| Apomixis | 1.00 |
| N | Cross (#) ♀ × ♂ | Viable Pollen with #colpi | Inviable Pollen with #colpi | Pollen Fertility | N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | (%) | |||
| Homoploid | (1) L. ovalifolium × L. ovalifolium | 0 | 0 | 2507 | 0 | 0 | 1 | 0 | 349 | 2 | 0 | 87.7 | 6 |
| (2) L. nydeggeri × L. ovalifolium | 0 | 0 | 2709 | 0 | 0 | 0 | 2 | 480 | 0 | 0 | 84.9 | 8 | |
| (3) L. nydeggeri × L. ovalifolium | 0 | 0 | 548 | 17 | 0 | 0 | 5 | 123 | 35 | 12 | 76.4 | 3 | |
| (4) L. ovalifolium × L. ovalifolium | 0 | 0 | 2386 | 0 | 0 | 0 | 1 | 556 | 0 | 0 | 81.1 | 7 | |
| (5) L. nydeggeri × L. ovalifolium | 1 | 1 | 2547 | 2 | 36 | 18 | 13 | 213 | 85 | 193 | 83.2 | 7 | |
| Heteroploid | (8) L. binervosum * × L. nydeggeri | 0 | 0 | 26 | 21 | 9 | 32 | 132 | 1150 | 250 | 27 | 3.4 | 4 |
| (11) L. binervosum * × L. nydeggeri | 0 | 0 | 0 | 0 | 0 | 8 | 31 | 317 | 91 | 0 | 0 | 6 | |
| (12) L. binervosum * × L. nydeggeri | 0 | 0 | 14 | 20 | 21 | 32 | 63 | 1593 | 442 | 53 | 2.5 | 4 | |
| (13) L. dodartii * × L. nydeggeri | 0 | 0 | 0 | 0 | 1 | 6 | 23 | 300 | 115 | 9 | 0.2 | 1 | |
| Cross (#) ♀ × ♂ | Number of Seeds | % Seed Germination (#) | |
|---|---|---|---|
| Homoploid | (1) L. ovalifolium × L. ovalifolium | 1 | 100 (1) |
| (2) L. nydeggeri × L. ovalifolium | 129 | 62 (80) | |
| (3) L. nydeggeri × L. ovalifolium | 149 | 90 (134) | |
| (4) L. ovalifolium × L. ovalifolium | 84 | 81 (68) | |
| (5) L. nydeggeri × L. ovalifolium | 107 | 84 (90) | |
| Heteroploid | (8) L. binervosum * × L. nydeggeri | 257 | 82 (210) |
| (11) L. binervosum * × L. nydeggeri | 320 | 33 (106) | |
| (12) L. binervosum * × L. nydeggeri | 170 | 65 (110) | |
| (13) L. dodartii * × L. nydeggeri | 100 | 94 (94) |
| Cross (#) ♀ × ♂ | Seedling Morphotypes #Cotyledons | Poly-Embryony | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Homoploid | (1) L. ovalifolium × L. ovalifolium | 0 | 100 (1) | 0 | 0 | 0 |
| (2) L. nydeggeri × L. ovalifolium | 0 | 100 (80) | 0 | 0 | 0 | |
| (3) L. nydeggeri × L. ovalifolium | 0 | 90 (120) | 6 (8) | 0 | 4 (6) | |
| (4) L. ovalifolium × L. ovalifolium | 0 | 97 (66) | 3 (2) | 0 | 0 | |
| (5) L. nydeggeri × L. ovalifolium | 1 (1) | 99 (89) | 0 | 0 | 0 | |
| Heteroploid | (8) L. binervosum * × L. nydeggeri | 0 | 83 (172) | 5 (10) | 0 | 12 (26) |
| (11) L. binervosum * × L. nydeggeri | 0 | 67 (71) | 3 (3) | 2 (2) | 28 (30) | |
| (12) L. binervosum * × L. nydeggeri | 0 | 77 (85) | 3 (3) | 4 (4) | 16 (18) | |
| (13) L. dodartii * × L. nydeggeri | 0 | 97 (91) | 1 (1) | 0 | 2 (2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, S.I.R.; Fernandes, J.; Borges da Silva, E.; Caperta, A.D. Reproductive Output and Insect Behavior in Hybrids and Apomicts from Limonium ovalifolium and L. binervosum Complexes (Plumbaginaceae) in an Open Cross-Pollination Experiment. Plants 2021, 10, 169. https://doi.org/10.3390/plants10010169
Conceição SIR, Fernandes J, Borges da Silva E, Caperta AD. Reproductive Output and Insect Behavior in Hybrids and Apomicts from Limonium ovalifolium and L. binervosum Complexes (Plumbaginaceae) in an Open Cross-Pollination Experiment. Plants. 2021; 10(1):169. https://doi.org/10.3390/plants10010169
Chicago/Turabian StyleConceição, Sofia I. R., Joana Fernandes, Elsa Borges da Silva, and Ana D. Caperta. 2021. "Reproductive Output and Insect Behavior in Hybrids and Apomicts from Limonium ovalifolium and L. binervosum Complexes (Plumbaginaceae) in an Open Cross-Pollination Experiment" Plants 10, no. 1: 169. https://doi.org/10.3390/plants10010169
APA StyleConceição, S. I. R., Fernandes, J., Borges da Silva, E., & Caperta, A. D. (2021). Reproductive Output and Insect Behavior in Hybrids and Apomicts from Limonium ovalifolium and L. binervosum Complexes (Plumbaginaceae) in an Open Cross-Pollination Experiment. Plants, 10(1), 169. https://doi.org/10.3390/plants10010169