Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review
Abstract
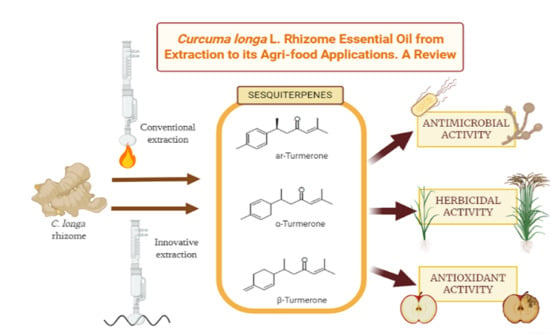
:1. Introduction
2. Extraction Methods to Obtain Essential Oil from C. longa Rhizomes
| Extraction method | Steam Distillation | Advantages |
| [63,64,65,71,74] |
| Limitations |
| [68,69,70] | ||
| Hydrodistillation | Advantages |
| [75,76] | |
| Limitations |
| [74,75] | ||
| Supercritical Fluid Extraction | Advantages |
| [73,79,83,84,85] | |
| Limitations |
| [79,82,83,88,89,91,92] | ||
| Subcritical Water Extraction | Advantages |
| [86,94] | |
| Limitations |
| [93] | ||
| Ultrasonic Extraction | Advantages |
| [68,82,95] | |
| Limitations | ||||
| Microwave Energy (SFME, MAE) | Advantages |
| [96,97,98] | |
| Limitations | ||||
| Solvent Extraction | Advantages |
| [99,100,101] | |
| Limitations |
3. Chemical Analysis of the Essential Oil Obtained from C. longa Rhizomes
| Part of Turmeric | Origin | Method of Extraction | Analysis | Yield | Main Components | Ref. |
|---|---|---|---|---|---|---|
| Powdered rhizomes | Nepal | Hydrodistillation Clevenger | GC-MS | 3.0% | β–turmerone (17.74%), α-turmeron (8.19%), epi-α-patschutene (7.19%), β–sesquiphellandrene (4.99%), 1,4-dimethyl-2-isobutylbenzene (4.4%) | [33] |
| Pulverized rhizome | India | Steam distillation + vacuum distillation | GC-MS | 1.6–46.6% | Turmerones, l-zingiberene, β–sesquiphellandrene, ar-curcumene | [72] |
| Rhizomes | Brazil | Hydrodistillation assisted by microwave (HDAM) | GC-MS | 0.6% | ar-turmerone (50.37 ± 0.99%), β–turmerone (14.39 ± 0.33%), ar-curcumene (6.24 ± 0.21%) | [98] |
| Rhizomes | Brazil | HDAM + Cryogenic grinding (CG) | GC-MS | 1.00% | ar-turmerone (47.97 ± 1.19%), β–turmerone (13.70 ± 0.55%), ar-curcumene (5.94 ± 0.27%) | [98] |
| Rhizomes | Brazil | Steam distillation assisted by microwave (SDAM) | GC-MS | 0.9% | - | [98] |
| Rhizomes | Brazil | SDAM + CG | GC-MS | 1.45% | - | [98] |
| Powdered dried rhizome | Serbia | Hydrodistillation Clevenger | GC-MS and GC-FID | 0.3 cm3/100 g | ar-turmerone (22.7%), turmerone (26%) and curlone (16.8%) | [104] |
| Rhizomes | Pakistan | Hydrodistillation | GC-MS | 0.673% | ar-turmerone (25.3%), α-turmerone (18.3%) and curlone (12.5%) | [158] |
| Powdered rhizomes | Thailand | Hydrodistillation Clevenger | GC-MS | - | ar-turmerone (43–49%), turmerone (13–16%) and curlone (17–18%) | [166,167] |
| Dried rhizomes | Brazil | SFE | GC-MS | 0.5–6.5 g/100 g | ar-turmerone (20%) and ar-, α- and β–turmerones (~75%) | [91] |
| Dried rhizomes | Brazil | Extraction with volatile solvents | GC-MS and CG-FID | 5.49% | α-turmerone and β –turmerone (~8.7%), ar-turmerone (~3.6%) | [104] |
| Dried rhizomes | Brazil | Steam distillation | GC-MS and CG-FID | 0.46% | ar-turmerone (~12.8%), α-turmerone and β –turmerone (~4.1%) | [104] |
| Dried rhizomes | China | Steam distillation | GC-MS | 4.50% w/w | ar-turmerone (11.81%) | [124] |
| Dried rhizomes | Nigeria | Hydrodistillation Clevenger | GC-MS | 1.33% w/w | ar-turmerone (44.4%), α-turmerone (20.8%), β–turmerone (26.5%) | [141] |
| Dry rhizomes | India | Hydrodistillation Clevenger | GC-MS | 2.9% | ar-turmerone (21.4%), α-santalene (7.2%) and ar-curcumene (6.6%) | [164] |
| Dried rhizomes | India | Hydrodistillation Clevenger | GC-MS | 3.05 ± 0.15% | ar-turmerone (30.3%), α-turmerone (26.5%), β–turmerone (19.1%) | [167] |
| Cured rhizomes | India | Hydrodistillation Clevenger | GC-MS | 4.45 ± 0.37% | ar-turmerone (28.3%), α-turmerone (24.8%), β–turmerone (21.1%) | [167] |
| Dried root | - | SFE | GC-MS | 2–5.3 wt% | ar-turmerone (31–67.1%), β–turmerone (2–37.9%), α-turmerone (0–21.3%) | [87] |
| Fresh rhizomes | Brazil | Hydrodistillation Clevenger | GC-MS | 1000 µL | α-turmerone (42.6%), β –turmerone (16.0%) and ar-turmerone (12.9%) | [34] |
| Fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 0.6–2.1% | Turmerone (35.24–44.22%) | [39] |
| Fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 0.8% | α-turmerone (44.1%), β–turmerone (18.5%) and ar-turmerone (5.4%) | [43] |
| Fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 0.36% | ar-turmerone (31.7%), α-turmerone (12.9%), β–turmerone (12.0%) and (Z)- β–ocimene (5.5%) | [44] |
| Fresh rhizomes | India | Modified distillation process | GC-MS | 2.09–2.50% | ar-turmerone (45.27%), curlone (5.6%), turmerone (4.4%), zingiberene (4.01%), ar-curcumene (4.01%), dehydrocurcumene (2.0%) | [73] |
| Fresh rhizome | Malaysia | SFE | GC-MS | - | ar-turmerone (10.84–21.50%), turmerone (36.14–45.68%) and curlone (21.27–22.30%) | [79] |
| Fresh rhizomes | Iran | SWE | GC-MS | 0.98% | ar-turmerone (62.88%), curcumin (10.49%), β–sesquiphellandrene (9.62%), α-phellandrene (6.50%) | [86] |
| Fresh rhizomes | Ecuador | Steam distillation | GC-FID and GC-MS | 0.8% v/w | ar-turmerone (45.5%) and α-turmerone (13.4%) | [105] |
| Fresh rhizomes | France | Steam distillation | GC-MS and GC-FTIR | 1.1% | α-turmerone (21.4%), zingiberene (11.8%), terpinolene (15.8%), β–sesquiphellandrene (8.8%), ar-turmerone (7.7%) and β–turmerone (7.1%) | [134] |
| Fresh mature rhizomes | Bhutan | Hydrodistillation Clevenger | GC-MS | 2–5.5% | α-turmerone (30–32%), ar-turmerone (17–26%) and β–turmerone (15–18%) | [139] |
| Fresh rhizome | India | Steam distillation | - | 2.03–6.50% | - | [156] |
| Fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 1.8–3.73 mL/plant | ar-turmerone (39.5–45.5%), curlone (9.8–11.7%), α-phellandrene (5.5–7.7%), eucalyptol (3.2–5.5%), β–himachalene (1.6–5.5%) and copen-11-ol (2.3–5.4%) | [157] |
| Fresh rhizomes | Nigeria | Hydrodistillation Clevenger | GC-MS | 10.5 g (0.7% w/w) | Turmerone (35.9%), α-phellandrene (15.5%), curlone (12.9%), 1,8-cineole (10.3%) and ar-turmerone (10.0%) | [157] |
| Fresh rhizomes | Brazil | Hydrodistillation Clevenger | GC-MS | 0.70% | Zingiberene (11%), sesquiphellandrene (10%), β–turmerone (10%) and α-curcumene (5%) | [159] |
| Mature fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 1.4% | ar-turmerone (24.4%), α-turmerone (20.5%) and β–turmerone (11.1%) | [166] |
| Fresh rhizomes | India | Hydrodistillation Clevenger | GC-MS | 3.52 ± 0.23% | α-turmerone (33.5%), ar-turmerone (21.0%), β–turmerone (18.9%) | [167] |
| Semi dried leaves and fresh rhizomes | India | Continuous water circulation with steam distillation | GC-MS | Leaves: 2.75–2.83% Rhizomes: 2.38–2.48% | Rhizome: Bisabolene (0.4%), ar-curcumene (2.3%), zingiberene (4.01%), dehydrocurcumene (2.0%), ar-turmerone (15.8%), turmerone (4.4%) and curlone (5.6%) | [71] |
| Semi-ripened and dried leaves | India | Water distillation techniques | GC-MS | 0.25–0.28% v/w | Terpinolene (33.0–57.6%), 1,8-cineole (1.9–7.9%), α-terpinene (1.7–3.9%), α-phellandrene (1.4–3.1%) | [36] |
| Partially senescenced leaves | India | Hydrodistillation Clevenger | GC-MS | - | α-phellandrene, p-cymene, α-terpinolene, 1,8-cineole, p-cymen-8-ol | [137] |
| Dried leaves | Nigeria | Hydrodistillation Clevenger | GC-MS | 0.67% w/w | ar-turmerone (63.4%), α-turmerone (13.7%), β–turmerone (12.6%) | [141] |
| Dried leaves | Nigeria | Hydrodistillation Clevenger | GC-MS | 0.67% w/w | ar-turmerone (63.4%), α-turmerone (13.7%), β–turmerone (12.6%) | [142] |
| Leaves | Bhutan | Hydrodistillation Clevenger | GC-MS | 0.3–0.42% | α-phellandrene (18.2%), 1,8-cineole (14.6%) and p-cymene (13.3%) | [139] |
| Leaves | India | Hydrodistillation Clevenger | GLC | 1.32% | α-phellandrene (38.24%), C8-aldehyde (20.58%), 1,8-cineole (8.64%), α-pinene (2.88%) and β–pinene (2.36%) | [140] |
| Leaves | Pakistan | Hydrodistillation Reverse dean-stark method | GC-MS | 145% | Eucalyptol (10.27%), β–pinene (3.57%), 2-methylisoborneol (2.91%), limonene (2.73%), β–phellandrene (2.49%) | [143] |
| Fresh leaves | India | Hydrodistillation Clevenger | GC-MS | 0.2–1.9% | α-Phellandrene (30.82–39.85%), terpinolene (25.74–26.59%) and eucalyptol (7.52–7.66%) | [39] |
| Fresh leaves | India | Hydrodistillation Clevenger | GC-MS | 0.65% | α-phellandrene (53.4%), terpinolene (11.5%) and 1,8-cineole (10.5%) | [43] |
| Fresh leaves | India | Hydrodistillation Clevenger | GC-MS | 0.53% | α-phellandrene (9.1%), terpinolene (8.8%), 1,8-cineole (7.3%) and undecanol (7.1%) | [44] |
| Roughly crushed fresh leaves | France | Steam distillation | GC-MS and GC-FTIR | 0.5% | Terpinolene (77%), 1,8-cineole (4.6%), α-terpinene (3.7%), α-phellandrene (2.8%), myrcene (1.4%) and δ-3-carene (1.1%) | [134] |
| Fresh leaves | India | Steam distillation | GC-MS | 0.15% | Terpinolene (71.2%), 1,8-cineole (6.2%), p-cymen-9-ol (4.2%) | [136] |
| Fresh leaves and stems | Colombia | Steam distillation | GC-MS | - | Turmerone (36.9%), α-turmerone (18.9%) and β–turmerone (13.6%) | [107] |
| Fresh aerial parts | India | SFE | GC-MS | 2.8% | p-cymene (25.4%), 1,8-cineole (18%), cis-sabinol (7.4%), β–pinene (6.3%) | [135] |
| Roughly crushed fresh flowers | France | Steam distillation | GC-MS and GC-FTIR | 0.15% | Terpinolene (67.4%), 1,8-cineole (4.6%), α-terpinene (4.4%), α-phellandrene (3.6%), myrcene (2%) and zingiberene (1.3%) | [134] |
4. Potential Applications of C. longa Essential Oil Obtained from Rhizomes in the Agri-Food Industry
4.1. Prevention and Inhibition of Microbial Attack in Crops and Food-Spoilage Microorganisms
4.2. Herbicidal Activity
4.3. Food Decay Prevention: Antioxidant Activity
| Antimicrobial Activity | |||
| Chemical Composition | Concentration | Effect | Ref. |
| 42.6% α-Turmerone 16.0% β-Turmerone 12.9% ar-Turmerone | 17.9 and 294.9 µg/mL | Decrease the development of Fusarium verticillioides by 56.0 and 79.3%, respectively, as well as the thickness and length of microconidia, fungal biomass and fumonisin production | [34] |
| 51.8% ar-Turmerone 11.9% ar-Turmerol | 1000 ppm | Complete mycelial growth inhibition of Colletotrichum falcatum and F. moniliforme | [192] |
| 51.8% ar-Turmerone 11.9% ar-Turmerol | 2000 ppm | Complete mycelial growth inhibition of Curvularia pallescens, Aspergillus niger and F. oxysporium | [192] |
| 58% ar-Turmerone Limonene Borneol | >45–90 µg/disc | Significant inhibition of Bacillus subtilis, Salmonella choleraesuis, Escherichia coli, A. niger and Saccharomyces cerivisiae at higher doses than chloramphenicol and amphotericin | [195] |
| 33.42% ar-Turmerone 22.35% α-Turmerone 20.14% β-Turmerone | 1–2% (v/v) | Antibacterial activity against E. coli and Staphylococcus aureus when incorporated to an edible film with sorbitol and egg white protein | [196] |
| - | 1–5% | Complete radial growth inhibition of C. gloeosporoides, Sphaceloma cardamomi, Pestalotia palmarum, Rhizoctonia solani, Aspergillus sp. and Fusarium sp. | [199] |
| 35.46% Turmerone 20.61% Cumene 13.82% ar-Turmerone | >0.5 µL | Antifungal effect against A. niger when incorporated to a biopolymer film | [202] |
| - | 0.5% w/w | Reduction of the growth of Penicillium and Cladosporium spp. in 60.3 and 41.6%, respectively, for 15 days when incorporated to an edible coating based on achira starch (Canna indica L.) | [203] |
| 33.2% ar-Turmerone 23.5% α-Turmerone 22.7% β-Turmerone | 0.10–0.5% v/v | Significant reduction of the growth rate of A. flavus, as well as complete inhibition of germination and sportulation | [211] |
| 53.10% ar-Turmerone | 2450 and 3300 µg/mL | Minimum inhibitory and minimum fungicidal concentration against F. graminearum | [215] |
| 53.10% ar-Turmerone | 3500 and 3000 µg/mL | Complete inhibition of fungal biomass and zearalenone production in F. graminearum, respectively | [215] |
| 20.4% α-Phellandrene 19.8% α-Turmerone 10.3% 1.8-Cineole 7.35% β-Turmerone | 0.06–0.36 µg/mL | One of the highest minimum inhibitory concentrations with respect to 11 different essential oils against five-food spoilage yeasts | [217] |
| Herbicidal Activity | |||
| 38.7% ar-Turmerone 18.6% β-Turmerone 14.2% α-Turmerone | 0.125–1 µL/mL | Reduction of the hypocotyl growth of Portulaca oleracea, Lolium multiflorum and Echinochloa crus-galli in 56.55, 40.45 and 39.33%, respectively, without affecting neither seed germination nor hycopotyl growth of tomato, cucumber and rice crops | [234] |
| 38.7% ar-Turmerone 18.6% β-Turmerone 14.2% α-Turmerone | 1 µL/mL | Significant inhibition of Cortaderia selloana seed germination (81.71%) | [234] |
| 38.7% ar-Turmerone 18.6% β-Turmerone 14.2% α-Turmerone | >0.125 µL/mL | Outstanding inhibitory effect in the development of C. selloana and Nicotiana glauca | [234] |
| Antioxidant Activity | |||
| 42.6% α-Turmerone 16.0% β-Turmerone 12.9% ar-Turmerone | IC50 10.03 mg/mL (DPPH) IC50 0.54 mg/mL (ABTS) | Dose-dependent DPPH and ABTS radical scavenging activities, as well as reducing power | [34] |
| 45.5% ar-Turmerone 13.4% α-Turmerone | IC50 14.5 ± 2.9 mg/mL (DPPH) 389.0 ± 12.0 µM Ascorbic Acid (AA) eq. | Low DPPH bleaching potential and ferric-reducing antioxidant power in comparison to Trolox | [105] |
| 24.4% ar-Turmerone 20.5% α-Turmerone 11.1% β-Turmerone | <100 Meq/kg (peroxide value) 0.04–0.08 TBA value 5–20 µL (DPPH) 10–100 µL (Fe2+ chelating effect) | The lowest peroxide value with respect to oleoresins, synthetic antioxidants and essential oil from dry rhizomes. More efficient inhibitory effect of malondialdehyde Higher DPPH radical scavenging, as well as Fe2+ chelating abilities than the dry ones (21.4% ar-turmerone, 7.2% α-santalene and 6.6% ar-curcumene) Higher DPPH radical scavenging activity than BHA and BHT | [164] |
| 21.4% ar-Turmerone 7.2% α-Santalene 6.6% ar-Curcumene | 100–200 Meq/kg (peroxide value) 0.04–0.08 TBA value 15–20 µL (DPPH) 10–100 µL (Fe2+ chelating effect) | Higher DPPH radical scavenging activity than BHA and BHT | [164] |
| 20.4% α-Phellandrene 19.8% α-Turmerone 10.3% 1,8-Cineole 7.35% β-Turmerone | 28.1 ± 1.45 mmol Trolox/L (PLC) | Free radical-scavenging potential twice higher than that of Trolox (~60 vs. 28.2%, respectively) Antioxidant activity (72.4%) near the values of the reference essential oil Thymus vulgaris (90.9%) and butylated hydroxyanisole (BHA) (86.74%) | [217] |
| 22.8% β-Sesquiphellandrene 9.5% Terpinolene | IC50 3.227 mg/mL (DPPH) IC50 1.541 mg/mL (ABTS) 1 mg/mL (antiperoxidative) | Hydrogen donating properties and reducing power. Potential option to prevent oxidative deterioration of fat containing food products | [256] |
| 35.46% Turmerone 20.61% Cumene 13.82% ar-Turmerone | 30 µL/mL | Suppression of oxidase enzyme activity of the fresh-cut “Fuji” apples by 9% when incorporated in a starch/carboxymethyl cellulose edible coating | [261] |
| 38.7% ar-Turmerone 14.2% α-Turmerone | 10 µL | Negligible DPPH radical scavenging activity with respect to other different essential oils (cinnamon, clove, green tea, lemon eucalyptus, rosemary, oregano and its main compound carvacrol) | [262] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singab, A.N.B.I. Medicinal & Aromatic Plants. Med. Aromat. Plants 2012, 1, 1000e109. [Google Scholar]
- Inoue, M.; Hayashi, S.; Craker, L.E. Role of medicinal and aromatic plants: Past, present, and future. In Pharmacognosy-Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019; pp. 13–26. [Google Scholar] [CrossRef] [Green Version]
- Saiz de Cos, P. Cúrcuma I (Curcuma longa L.). Reduca Ser. Botánica 2014, 7, 84–99. [Google Scholar]
- Araújo, C.A.C.; Leon, L.L. Biological activities of Curcuma longa L. Memórias Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.M.; Singh, R.; Chandra, R. Therapeutic uses of Curcuma longa (turmeric). Indian J. Clin. Biochem. 2001, 16, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of turmeric (Curcuma longa) on skin health: A systematic review of the clinical evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Dasgupta, A. Antiinflammatory herbal supplements. In Translational Inflammation; Actor, J.K., Smith, K.C., Eds.; Elsevier Inc.: London, UK, 2019; pp. 69–91. [Google Scholar] [CrossRef]
- El-Kenawy, A.E.-M.; Hassan, S.M.A.; Mohamed, A.M.M.; Mohammed, H.M.A.M. Tumeric or Curcuma longa Linn. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, A., Eds.; Elsevier Inc.: London, UK, 2019; pp. 447–453. [Google Scholar] [CrossRef]
- Ortega, A.M.M.; Segura Campos, M.R. Medicinal plants and their bioactive metabolites in cancer prevention and treatment. In Bioactive Compounds: Health Benefits and Potential Applications; Campos, M.R.S., Ed.; Elsevier Inc.: Duxford, UK, 2019; pp. 83–109. [Google Scholar] [CrossRef]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Future J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef]
- Alvis, A.; Arrazola, G.; Martínez, W. Evaluation of antioxidant activity and potential hydro-alcoholic extracts of curcuma (Curcuma longa). Inf. Tecnol. 2012, 23, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Sawant, R.S.; Godghate, A.G. Qualitative phytochemical screening of rhizomes of Curcuma longa Linn. Int. J. Sci. Environ. Technol. 2013, 2, 634–641. [Google Scholar]
- Abraham, A.; Samuel, S.; Mathew, L. Pharmacognostic evaluation of Curcuma longa L. rhizome and standardization of its formulation by HPLC using curcumin as marker. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 38–42. [Google Scholar]
- Meng, F.; Zhou, Y.; Ren, D.; Wang, R. Turmeric: A review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: London, UK, 2018; pp. 299–350. [Google Scholar] [CrossRef]
- Carballido, E. Características de la Planta de la Cúrcuma. Available online: https://www.botanical-online.com/plantas-medicinales/curcuma-caracteristicas (accessed on 18 November 2020).
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Aggarwal, B.B. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops 2011, 2, 28–54. [Google Scholar] [CrossRef]
- González-Albadalejo, J.; Sanz, D.; Claramunt, R.M.; Lavandera, J.L.; Alkorta, I.; Elguero, J. Curcumin and curcuminoids: Chemistry, structural studies and biological properties. An. Real Acad. Nac. Farm. 2015, 81, 278–310. [Google Scholar]
- Ramesh, T.N.; Paul, M.; Manikanta, K.; Girish, K.S. Structure and morphological studies of curcuminoids and curcuminoid mixture. J. Cryst. Growth 2020, 547, 125812. [Google Scholar] [CrossRef]
- Bambirra, M.L.A.; Junqueira, R.G.; Glória, M.B. Influence of post harvest processing conditions on yield and quality of ground turmeric (Curcuma longa L.). Braz. Arch. Biol. Technol. 2002, 45, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Chin. Med. Sci. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengmark, S.; Mesa, M.D.; Gil, A. Plant-derived health: The effects of turmeric and curcuminoids. Nutr. Hosp. 2009, 24, 273–281. [Google Scholar] [PubMed]
- Tayyem, R.F.; Heath, D.D.; Al-delaimy, W.K.; Rock, C.L.; Tayyem, R.F.; Heath, D.D.; Al-delaimy, W.K.; Rock, C.L.; Tayyem, R.F.; Heath, D.D.; et al. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Akbar, A.; Kuanar, A.; Joshi, R.K.; Sandeep, I.S.; Mohanty, S. Development of prediction model and experimental validation in predicting the curcumin content of turmeric (Curcuma longa L.). Front. Plant Sci. 2016, 7, 1507. [Google Scholar] [CrossRef] [Green Version]
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Curcuma longa, the polyphenolic curcumin compound and pharmacological effects on liver. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2019; pp. 125–134. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Changtam, C.; De Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 2010, 45, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [PubMed]
- Arshad, L.; Nasir, S.; Bukhari, A.; Jantan, I. An overview of structure-activity relationship studies of curcumin analogs as antioxidant and anti-inflammatory agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, S.A.; El-shishtawy, R.M.; Al-footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Benzaria, A.; Maresca, M.; Taieb, N.; Dumay, E. Interaction of curcumin with phosphocasein micelles processed or not by dynamic high-pressure. Food Chem. 2013, 138, 2327–2337. [Google Scholar] [CrossRef]
- Devkota, L.; Rajbhandari, M. Composition of essential oils in turmeric rhizome. Nepal J. Sci. Technol. 2015, 16, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; Santos, P.A.D.S.R.D.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Filho, B.A.A.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Ferreira Guimares, A.; Andrade Vinhas, A.C.; Ferraz Gomes, A.; Souza, L.H.; Baier Krepsky, P. Essential oil of Curcuma longa L. rhizomes chemical composition, yield variation and stability. Quim. Nova 2020, 43, 909–913. [Google Scholar] [CrossRef]
- Babu, G.D.K.; Shanmugam, V.; Ravindranath, S.D.; Joshi, V.P. Comparison of chemical composition and antifungal activity of Curcuma longa L. leaf oils produced by different water distillation techniques. Flavour Fragr. J. 2007, 22, 191–196. [Google Scholar] [CrossRef]
- Usman, L.A.; Hamid, A.A.; George, O.C.; Ameen, O.M.; Muhammad, N.O.; Zubair, M.F.; Lawal, A. Chemical composition of rhizome essential oil of Curcuma longa L. growing in North Central Nigeria. World J. Chem. 2009, 4, 178–181. [Google Scholar]
- Niranjan, A.; Singh, S.; Dhiman, M.; Tewari, S.K. Biochemical composition of Curcuma longa L. accessions. Anal. Lett. 2013, 46, 1069–1083. [Google Scholar] [CrossRef]
- Akbar, A.; Kuanar, A.; Sandeep, I.S.; Kar, B.; Singh, S.; Mohanty, S.; Patnaik, J.; Nayak, S. GC-MS analysis of essential oil of some high drug yielding genotypes of turmeric (Curcuma longa L.). Int. J. Pharm. Pharm. Sci. 2015, 7, 35–40. [Google Scholar]
- Sandeep, I.S.; Das, S.; Nayak, S.; Mohanty, S. Chemometric profile of Curcuma longa L. towards standardization of factors for high essential oil yield and quality. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 949–957. [Google Scholar] [CrossRef]
- Choudhary, V.K.; Kumar, P.S. Weed suppression, nutrient leaching, water use and yield of turmeric (Curcuma longa L.) under different land configurations and mulches. J. Clean. Prod. 2019, 210, 795–803. [Google Scholar] [CrossRef]
- Leela, N.K.; Tava, A.; Shafi, P.M.; Sinu, P.J.; Chempakam, B. Chemical composition of essential oils of turmeric. Acta Pharm. 2002, 52, 137–141. [Google Scholar]
- Raina, V.K.; Srivastava, S.K.; Syamsundar, K.V. Rhizome and leaf oil composition of Curcuma longa from the lower himalayan region of northern India. J. Essent. Oil Res. 2005, 17, 556–559. [Google Scholar] [CrossRef]
- Awasthi, P.; Dixit, S. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of Northern India. J. Young Pharm. 2009, 1, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Panda, M.K.; Subudhi, E.; Nayak, S. Chemical composition of leaf and rhizome oil of an elite genotype Curcuma longa L. from South Eastern ghats of Orissa. J. Pharm. Res. 2010, 3, 1630–1633. [Google Scholar]
- Mishra, R.; Gupta, A.K.; Kumar, A.; Lal, R.K.; Saikia, D.; Chanotiya, C.S. Genetic diversity, essential oil composition, and in vitro antioxidant and antimicrobial activity of Curcuma longa L. germplasm collections. J. Appl. Res. Med. Aromat. Plants 2018, 10, 75–84. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Suh, Y.; Lee, H.; Lee, Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int. J. Mol. Med. 2013, 31, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankasem, M.; Wuthi-udomlert, M.; Gritsanapan, W. Antidermatophytic properties of ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol. 2013, 2013, 250597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, G.G.L.; Kwok, H.F.; Lee, J.K.M.; Jiang, L.; Chan, K.M.; Cheng, L.; Wong, E.C.W.; Leung, P.C.; Fung, K.P.; Lau, C.B.S. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J. Funct. Foods 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Orellana-paucar, A.M.; Afrikanova, T.; Thomas, J.; Aibuldinov, Y.K.; Dehaen, W.; De Witte, P.A.M.; Esguerra, C.V. Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS ONE 2013, 8, e81634. [Google Scholar] [CrossRef]
- Hucklenbroich, J.; Klein, R.; Neumaier, B.; Graf, R.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014, 5, 100. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, Z.; Li, P.; Yan, L.; Zhou, W.; Tang, Y. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- Khayreddine, B. Essential Oils, An Alternative to Synthetic Food Additives and Thermal Treatments; MedCrave Group LLC: Edmond, OK, USA, 2018; pp. 1–44. [Google Scholar]
- Boskovic, M.; Baltic, Z.M.; Ivanovic, J.; Duric, J.; Loncina, J.; Dokmanovic, M.; Markovic, R. Use of essential oils in order to prevent foodborne illnesses caused by pathogens in meat. Tehnol. Mesa 2013, 54, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Mihai, A.L.; Popa, M.E. Essential oils utilization in food industry—A literature review. Sci. Bull. Ser. F Biotechnol. 2013, 17, 187–192. [Google Scholar]
- Mihai, A.L.; Popa, M.E. Inhibitory effects of essential oils with potential to be used in food industry. Sci. Pharm. 2014, 18, 220–225. [Google Scholar]
- Bhavaniramya, S.; Vishnupriya, S.; Al-aboody, M.S. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice). Appl. Sci. 2020, 10, 5581. [Google Scholar] [CrossRef]
- Ibáñez, M.D. Commercial Essential Oils: Sustainable Alternatives in the Agri-Food Industry. Ph.D. Thesis, Universitat de València, Valencia, Spain, 2019. [Google Scholar]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry. A review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Weiss, E.A. Turmeric. In Spice Crops; CABI Publishing: Wallingford, CT, USA, 2002; pp. 338–352. [Google Scholar]
- Balakrishan, K. Postharvest technology and processing of turmeric. In Turmeric. The Genus Curcuma; Ravindran, P., Nirman Babu, K., Sivaraman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 193–256. [Google Scholar]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Sorensen, E. Principles of Binary Distillation. In Distillation: Fundamentals and Principles; Górak, A., Sorensen, E., Eds.; Elsevier: Oxford, UK, 2014; pp. 145–186. [Google Scholar]
- Akdag, A.; Ozturk, E. Distillation methods of essential oils. Nisan 2019, 45, 22–31. [Google Scholar]
- Hielscher Ultrasonic Hydrodistillation of Essential Oils. Available online: https://www.hielscher.com/ultrasonic-hydrodistillation-of-essential-oils.htm (accessed on 5 July 2020).
- Gujarathi, D.B.; Ilay, N.T. Continuous water circulation distillation: A modification of steam distillation. J. Chem. Educ. 1993, 70, 86. [Google Scholar] [CrossRef]
- Santos, D.T.; Angela, A.; Meireles, M. Extraction of volatile oils by supercritical fluid extraction: Patent survey. Recent Patents Eng. 2011, 5, 17–22. [Google Scholar] [CrossRef]
- Chandra, A.; Prajapati, S.; Garg, S.K.; Rathore, A.K. Extraction of turmeric oil by continuous water circulation distillation. Int. J. Sci. Eng. Appl. Sci. 2016, 2, 2395–3470. [Google Scholar]
- Matsumura, S.; Murata, K.; Zaima, N.; Yoshioka, Y.; Morimoto, M.; Kugo, H.; Yamamoto, A.; Moriyama, T.; Matsuda, H. Inhibitory activities of essential oil obtained from turmeric and its constituents against β-secretase. Nat. Prod. Commun. 2016, 11, 1785–1788. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, H.; Jain, T.; Malik, N.; Chandra, A.; Singh, S. Isolation and chemical analysis of turmeric oil from rhizomes. In Proceedings of the Chemical Engineering Towards Sustainable Development, Chennai, India, 27–30 December 2016; pp. 1–14. [Google Scholar]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Silva, S.G.; da Cruz, J.N.; Ortiz, E.; da Costa, W.A.; Bezerra, F.W.F.; Cunha, V.M.B.; Cordeiro, R.M.; Al, E. Supercritical CO2 application in essential oil extraction. In Industrial Applications of Green Solvents: Volume II; Inamuddin, D., Mobin, R., Asiri, A.M., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2019; pp. 1–28. [Google Scholar]
- Silva, L.V.; Nelson, D.L.; Drummond, M.F.B.; Dufossé, L.; Glória, M.B.A. Comparison of hydrodistillation methods for the deodorization of turmeric. Food Res. Int. 2005, 38, 1087–1096. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Tran, T.H.; Nguyen, P.T.N.; Pham, T.N.; Nguyen, D.C.; Dao, T.P.; Nguyen, T.D.; Nguyen, D.H.; Vo, D.V.N.; Le, X.T.; Le, N.T.H.; et al. Green technology to optimize the extraction process of turmeric (Curcuma longa L.) oils. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sanya, China, Sanya, China, 17–19 November, 2018; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 479, pp. 1–6. [Google Scholar] [CrossRef]
- Haiyee, Z.A.; Shah, S.H.M.; Ismail, K.; Hashim, N.; Ismail, W.I.W. Quality parameters of Curcuma longa L. extracts by supercritical fluid extraction (SFE) and ultrasonic assisted extraction (UAE). Malays. J. Anal. Sci. 2016, 20, 626–632. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: A comparative study. Pak. J. Biotechnol. 2016, 13, 173–180. [Google Scholar]
- Lee, W.-J.; Suleiman, N.; Hadzir, N.H.N.; Chong, G.-H. Supercritical fluids for the extraction of oleoresins and plant phenolics. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, D., Asiri, A.M., Isloor, A.M., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 279–328. [Google Scholar] [CrossRef]
- Khezeli, T.; Ghaedi, M.; Bahrani, S.; Daneshfar, A. Supercritical fluid extraction in separation and preconcentration of organic and inorganic species. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Soylak, M., Yilmaz, E., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 425–451. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical fluid extraction of bioactive compounds from plant materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Mottahedin, P.; Asl, A.H.; Khajenoori, M. Extraction of curcumin and essential oil from Curcuma longa L. by subcritical water via response surface methodology. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Khanam, S. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Chang, L.H.; Jong, T.T.; Huang, H.S.; Nien, Y.F.; Chang, C.M.J. Supercritical carbon dioxide extraction of turmeric oil from Curcuma longa Linn and purification of turmerones. Sep. Purif. Technol. 2006, 47, 119–125. [Google Scholar] [CrossRef]
- Kao, L.; Chen, C.R.; Chang, C.M.J. Supercritical CO2 extraction of turmerones from turmeric and high-pressure phase equilibrium of CO2+ turmerones. J. Supercrit. Fluids 2007, 43, 276–282. [Google Scholar] [CrossRef]
- Carvalho, P.I.N.; Osorio-Tobón, F.J.; Rostagno, M.A.; Mauricio, A.; Petenate, A.J.; Meireles, A.A. Optimization of the ar-turmerone extraction from turmeric (Curcuma longa L.) using supercritical carbon dioxide. In Proceedings of the 14th European Meeting on Supercritical Fluids, Marselha, France, 18–21 May 2014. [Google Scholar]
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54. [Google Scholar] [CrossRef]
- Topiar, M.; Sajfrtova, M.; Karban, J.; Sovova, H. Fractionation of turmerones from turmeric SFE isolate using semi-preparative supercritical chromatography technique. J. Ind. Eng. Chem. 2019, 77, 223–229. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Baysal, T.; Demirdoven, A. Ultrasound in food technology. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 163–182. [Google Scholar]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa-Morales, M.E.; López-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Hassan, J.; Osman, N.H.; Abbas, Z. Extraction of essential oils from Zingiberaceace family by using solvent-free microwave extraction (SFME), microwave-assisted extraction (MAE) and hydrodistillation (HD). Asian J. Appl. Sci. 2017, 5, 97–100. [Google Scholar]
- Akloul, R.; Benkaci-Ali, F.; Eppe, G. Kinetic study of volatile oil of Curcuma longa L. rhizome and Carum carvi L. fruits extracted by microwave-assisted techniques using the cryogrinding. J. Essent. Oil Res. 2014, 26, 473–485. [Google Scholar] [CrossRef]
- Ching, W.-Y.; Bin-Yusoff, Y.; Wan-Amarina, W.-N.B. Extraction of essential oil from Curcuma longa. J. Food Chem. Nutr. 2014, 2, 1–10. [Google Scholar]
- Hafizuddin Mokhtar, M. Extraction of Essential Oil from Turmeric (Curcuma longa). Bachelor’s Thesis, Universiti Teknologi MARA, Selangor, Malaysia, 2017. [Google Scholar]
- Zhilyakova, E.; Novikov, O.; Pisarev, D.; Boyko, N.; Nikitin, K. Prospects of using freons as extractants of Curcuma longa L. root essential oil. Adv. Biol. Sci. Res. 2019, 7, 373–376. [Google Scholar] [CrossRef]
- Shang, Z.-P.; Xu, L.-L.; Lu, Y.-Y.; Guan, M.; Li, D.-Y.; Le, Z.-Y.; Bai, Z.-L.; Qiao, X.; Ye, M. Advances in chemical constituents and quality control of turmeric. World J. Tradit. Chin. Med. 2019, 5, 116–121. [Google Scholar] [CrossRef]
- Manzan, A.C.C.M.; Toniolo, F.S.; Bredow, E.; Povh, N.P. Extraction of essential oil and pigments from Curcuma longa [L.] by steam distillation and extraction with volatile solvents. J. Agric. Food Chem. 2003, 51, 6802–6807. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, J.; Stanojevic, L.; Cvetkovic, D.; Danilovic, B. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.). Adv. Technol. 2015, 4, 19–25. [Google Scholar] [CrossRef]
- Pino, J.A.; Fon-fay, F.M.; Falco, A.S.; Rodeiro, I. Chemical composition and biological activities of essential oil from turmeric (Curcuma longa L.) rhizomes grown in Amazonian Ecuador. Rev. CENIC 2018, 49, 1–8. [Google Scholar]
- Afzal, A.; Oriqat, G.; Khan, M.A.; Jose, J.; Afzal, M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J. Biol. Act. Prod. Nat. 2013, 3, 1–55. [Google Scholar] [CrossRef]
- Coy Barrera, C.C.A.; Eunice Acosta, G. Antibacterial activity and chemical composition of essential oils of rosemary (Rosemary officinalis), thyme (Thymus vulgaris) and turmeric (Curcuma longa) from Colombia. Rev. Cuba. Plantas Med. 2013, 18, 237–246. [Google Scholar]
- Jain, V.; Prasad, V.; Singh, S.; Pal, R. HPTLC method for the quantitative determination of ar-turmerone and turmerone in lipid soluble fraction from Curcuma longa. Nat. Prod. Commun. 2007, 2, 927–932. [Google Scholar] [CrossRef]
- Bahl, J.R.; Bansal, R.P.; Garg, S.N.; Gupta, M.M.; Singh, V.; Goel, R.; Kumar, S. Variation in yield of curcumin and yield and quality of leaf and rhizome essential oils among Indian land races of turmeric Curcuma longa L. Proc. Indian Natl. Sci. Acad. 2014, 80, 143–156. [Google Scholar] [CrossRef]
- Wegmann, M.; Lersch, P.; Wenk, H.H.; Klee, S.K.; Maczkiewitz, U.; Farwick, M.; Goldschmidt, E. Protective turmerones from Curcuma longa. Pers. Care 2009, 37–40. [Google Scholar]
- Bagad, A.S.; Joseph, J.A.; Bhaskaran, N.; Agarwal, A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Adv. Pharmacol. Sci. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Del Prete, D.; Millán, E.; Pollastro, F.; Chianese, G.; Luciano, P.; Collado, J.A.; Munoz, E.; Appendino, G.; Taglialatela-Scafati, O. Turmeric sesquiterpenoids: Expeditious resolution, comparative bioactivity, and a new bicyclic turmeronoid. J. Nat. Prod. 2016, 79, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran Nair, K. Turmeric (Curcuma longa L.) and ginger (Zingiber o cinale Rosc.)—World’s Invaluable Medicinal Spices the Agronomy and Economy of Turmeric; Springer: Cham, Switzerland, 2019; pp. 1–568. [Google Scholar]
- Murakami, A.; Furukawa, I.; Miyamoto, S.; Tanaka, T.; Ohigashi, H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. BioFactors 2013, 39, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.M.; Hon, P.M.; Lee, M.Y.H.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Jiang, L.; Kwok, H.F.; Lee, J.K.M.; Chan, K.M.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin—The importance of turmerones. J. Funct. Foods 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Lee, H.-S. Antimicrobial properties of turmeric (Curcuma longa L.) rhizome-derived ar-turmerone and curcumin. Food Sci. Biotechnol. 2006, 15, 559–563. [Google Scholar]
- Lee, H.S. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006, 97, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.U.; Jung, S.H.; Ahn, B.Z. Recognition of pharmacophore of ar-turmerone for its anticancer activity. Arch. Pharm. Res. 1993, 16, 254–256. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Komiya, T.; Moteki, H.; Katsuzaki, H.; Imai, K.; Hibasami, H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002, 9, 481–484. [Google Scholar] [CrossRef]
- Cheng, S.B.; Wu, L.C.; Hsieh, Y.C.; Wu, C.H.; Chan, Y.J.; Chang, L.H.; Chang, C.M.J.; Hsu, S.L.; Teng, C.L.; Wu, C.C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. Induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J. Agric. Food Chem. 2012, 60, 9620–9630. [Google Scholar] [CrossRef]
- Chen, M.; Chang, Y.Y.; Huang, S.; Xiao, L.H.; Zhou, W.; Zhang, L.Y.; Li, C.; Zhou, R.P.; Tang, J.; Lin, L.; et al. Aromatic-turmerone attenuates LPS-induced neuroinflammation and consequent memory impairment by targeting TLR4-dependent signaling pathway. Mol. Nutr. Food Res. 2018, 62, 1700281. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Jiao, J.; Jiao, L. Ar-turmerone exerts anti-proliferative and anti-inflammatory activities in HaCaT keratinocytes by inactivating hedgehog pathway. Inflammation 2020, 43, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Z.; Wei, J.; Su, P.; Chen, D.; Pan, W.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; et al. Contrastive analysis of chemical composition of essential oil from twelve Curcuma species distributed in China. Ind. Crops Prod. 2017, 108, 17–25. [Google Scholar] [CrossRef]
- Devi, L.R.; Rana, V.S.; Devi, S.I.; Blázquez, M.A.; Devi, L.R.; Rana, V.S.; Devi, S.I. Chemical composition and antimicrobial activity of the essential oil of Curcuma leucorhiza Roxb. J. Essent. Oil Res. 2012, 24, 533–538. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evidence-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Singh, S.; Kapoor, I.P.S.; Singh, G.; Isidorov, V.; Szczepaniak, L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013, 3, 42–48. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Banerjee, A.; Sahoo, A.; Nasim, N.; Sahoo, S.; Kar, B.; Patnaik, J.; Panda, P.C.; Nayak, S. Chemical composition and antioxidant activity of essential oil from leaves and rhizomes of Curcuma angustifolia Roxb. Nat. Prod. Res. 2017, 31, 2188–2191. [Google Scholar] [CrossRef]
- Zhu, J.; Lower-Nedza, A.D.; Hong, M.; Jiec, S.; Wang, Z.; Yingmao, D.; Tschiggerl, C.; Bucar, F.; Brantner, A.H. Chemical composition and antimicrobial activity of three essential oils from Curcuma wenyujin. Nat. Prod. Commun. 2013, 8, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.L.; Lee, G.S.; Rahman, S.N.S.A.; Hamdi, O.A.A.; Awang, K.; Nugroho, N.A.; Malek, S.N.A. Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. Sci. World J. 2014, 2014, 397430. [Google Scholar] [CrossRef] [Green Version]
- König, W.A.; Rieck, A.; Hardt, I.; Gehrcke, B.; Kubeczka, K.-H.; Muhle, H. Enantiomeric composition of the chiral constituents of essential oils. Part 2: Sesquiterpene hydrocarbons. J. High Resolut. Chromatogr. 1994, 17, 315–320. [Google Scholar] [CrossRef]
- Chizzola, R. Regular monoterpenes and sesquiterpenes. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Heidelberg, Germany, 2013; pp. 2973–3008. [Google Scholar] [CrossRef]
- Tamta, A.; Prakash, O.; Punetha, H.; Pant, A.K. Chemical composition and in vitro antioxidant potential of essential oil and rhizome extracts of Curcuma amada Roxb. Cogent Chem. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Chane-Ming, J.; Vera, R.; Chalchat, J.C.; Cabassu, P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251. [Google Scholar] [CrossRef]
- Garg, S.N.; Mengi, N.; Patra, N.K.; Charles, R.; Kumar, S. Chemical examination of the leaf essential oil of Curcuma longa L. from the North Indian plains. Flavour Fragr. J. 2002, 17, 103–104. [Google Scholar] [CrossRef]
- Pande, C.; Chanotiya, C.S. Constituents of the leaf oil of Curcuma longa L. from Uttaranchal. J. Essent. Oil Res. 2006, 18, 166–167. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, S.; Tewari, S.K. Study of leaf oil composition from various accessions of Curcuma longa L. grown on partially reclaimed sodic soil. Int. J. Plant Environ. 2019, 5, 293–296. [Google Scholar] [CrossRef]
- Bansal, R.P.; Bahl, J.R.; Garg, S.N.; Naqvi, A.A.; Kumary, S. Differential chemical compositions of the essential oils of the shoot organs, rhizomes and rhizoids in the turmeric Curcuma longa grown in indo-gangetic plains. Pharm. Biol. 2002, 40, 384–389. [Google Scholar] [CrossRef]
- Sharma, R.K.; Misra, B.P.; Sarma, T.C.; Bordoloi, A.K.; Pathak, M.G.; Leclercq, P.A. Essential oils of Curcuma longa L. from Bhutan. J. Essent. Oil Res. 1997, 9, 589–592. [Google Scholar] [CrossRef]
- Behura, S.; Sahoo, S.; Srivastava, V.K. Major constituents in leaf essential oils of Curcuma longa L. and Curcuma aromatica Salisb. Curr. Sci. 2002, 83, 1312–1313. [Google Scholar]
- Ajaiyeoba, E.O.; Sama, W.; Essien, E.E.; Olayemi, J.O.; Ekundayo, O.; Walker, T.M.; Setzer, W.N. Larvicidal activity of turmerone-rich essential oils of Curcuma longa leaf and rhizome from Nigeria on Anopheles gambiae. Pharm. Biol. 2008, 46, 279–282. [Google Scholar] [CrossRef]
- Essien, E.; Newby, J.; Walker, T.; Setzer, W.; Ekundayo, O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in Southern Nigeria. Medicines 2015, 2, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Ananya, K.; Sujata, M.; Pande, M.K.; Sanghamitra, N. Essential oils from leaves of micropropagated turmeric. Curr. Sci. 2009, 96, 1166–1167. [Google Scholar]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Cuticular damage of Lucilia cuprina larvae exposed to Curcuma longa leaves essential oil and its major compound α-phellandrene. Data Br. 2018, 21, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, A.; Gomes, E.N.; Richardi, V.S.; Martins, C.E.N.; Brum, J.S.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Essential oil from Curcuma longa leaves: Can an overlooked by-product from turmeric industry be effective for myiasis control? Ind. Crops Prod. 2019, 132, 352–364. [Google Scholar] [CrossRef]
- Sindhu, S.; Chempakam, B.; Leela, N.K.; Bhai, R.S. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem. Toxicol. 2011, 49, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Parveen, Z.; Nawaz, S.; Siddique, S.; Shahzad, K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Indian J. Pharm. Sci. 2013, 75, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Kulpapangkorn, W.; Mai-Leang, S. Effect of plant nutrition on turmeric production. Procedia Eng. 2012, 32, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the volatile compositions of Curcuma species. Foods 2019, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Lal, R.K.; Chanotiya, C.S.; Shanker, K.; Gupta, P.; Shukla, S. Prediction of genetic variability and character contribution using path analysis in turmeric (Curcuma longa L.) germplasm. Trends Phytochem. Res. 2019, 3, 91–100. [Google Scholar]
- Chowdhury, J.U.; Nandi, N.C.; Bhuiyan, M.N.I.; Mobarok, M.H. Essential oil constituents of the rhizomes of two types of Curcuma longa of Bangladesh. Bangladesh J. Sci. Ind. Res. 2008, 43, 259–266. [Google Scholar] [CrossRef]
- Pal, K.; Chowdhury, S.; Dutta, S.K.; Chakraborty, S.; Chakraborty, M.; Pandit, G.K.; Dutta, S.; Paul, P.K.; Choudhury, A.; Majumder, B.; et al. Analysis of rhizome colour content, bioactive compound profiling and ex-situ conservation of turmeric genotypes (Curcuma longa L.) from sub-Himalayan terai region of India. Ind. Crops Prod. 2020, 150, 112401. [Google Scholar] [CrossRef]
- Kumar Rai, S.; Kumar Rai, K.; Pandey, N.; Kumari, A.; Tripathi, D.; Shashi Pandey, R. Varietal performance of turmeric (Curcuma longa L.) with special reference to curcumin and essential oil content under climatic conditions of Indogangetic plains. Veg. Sci. 2016, 43, 36–43. [Google Scholar]
- Shashidhar, M.D.; Hegde, N.K.; Hiremath, J.S.; Kukunoor, J.S.; Srikantprasad, D.; Patil, R.T. Evaluation of turmeric (Curcuma longa L.) genotypes for yield, curcumin and essential oil content in northern dry zone of Karnataka. J. Pharmacogn. Phytochem. 2018, SP3, 130–134. [Google Scholar]
- Sahoo, A.; Kar, B.; Jena, S.; Dash, B.; Ray, A.; Sahoo, S.; Nayak, S. Qualitative and quantitative evaluation of rhizome essential oil of eight different cultivars of Curcuma longa L. (Turmeric). J. Essent. Oil-Bear. Plants 2019, 22, 239–247. [Google Scholar] [CrossRef]
- Plotto, A. Turmeric: Post-Production Management; Mazaud, F., Röttger, A., Steffel, K., Eds.; Food and Agricultural Organization of the United Nations (FAO): Rome, Italy, 2004; pp. 1–21. [Google Scholar]
- Oyemitan, I.A.; Elusiyan, C.A.; Onifade, A.O.; Akanmu, M.A.; Oyedeji, A.O.; McDonald, A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in Southwest Nigeria. Toxicol. Rep. 2017, 4, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ilyas, S.; Parveen, Z.; Javed, S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010, 22, 3153–3158. [Google Scholar]
- Gonçalves, G.M.S.; Barros, P.P.; da Silva, G.H.; Fedes, G.R. The essential oil of Curcuma longa rhizomes as an antimicrobial and its composition by Gas Chromatography/Mass Spectrometry. Rev. Ciências Médicas 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Sandeep, I.; Sanghamitra, N.; Sujata, M. Differential effect of soil and environment onmetabolic expression of tumeric. Indian J. Exp. Biol. 2015, 53, 406–411. [Google Scholar]
- Sandeep, I.S.; Kuanar, A.; Akbar, A.; Kar, B.; Das, S.; Mishra, A.; Sial, P.; Naik, P.K.; Nayak, S.; Mohanty, S. Agroclimatic zone based metabolic profiling of turmeric (Curcuma longa L.) for phytochemical yield optimization. Ind. Crops Prod. 2016, 85, 229–240. [Google Scholar] [CrossRef]
- Akbar, A.; Kuanar, A.; Patnaik, J.; Mishra, A.; Nayak, S. Application of artificial neural network modeling for optimization and prediction of essential oil yield in turmeric (Curcuma longa L.). Comput. Electron. Agric. 2018, 148, 160–178. [Google Scholar] [CrossRef]
- Garg, S.N.; Bansal, R.P.; Gupta, M.M.; Kumar, S. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of North Indian plains. Flavour Fragr. J. 1999, 14, 315–318. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food Chem. Toxicol. 2010, 48, 1026–1031. [Google Scholar] [CrossRef]
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Monton, C.; Luprasong, C.; Charoenchai, L. Convection combined microwave drying affect quality of volatile oil compositions and quantity of curcuminoids of turmeric raw material. Rev. Bras. Farm. 2019, 29, 434–440. [Google Scholar] [CrossRef]
- Monton, C.; Luprasong, C.; Charoenchai, L. Acceleration of turmeric drying using convection and microwave-assisted drying technique: An optimization approach. J. Food Process. Preserv. 2019, 43, e14096. [Google Scholar] [CrossRef]
- El-Hawaz, R.F.; Grace, M.H.; Janbey, A.; Lila, M.A.; Adelberg, J.W. In vitro mineral nutrition of Curcuma longa L. affects production of volatile compounds in rhizomes after transfer to the greenhouse. BMC Plant Biol. 2018, 18, 122. [Google Scholar] [CrossRef]
- De Ferrari, M.P.S.; dos Queiroz, M.S.; de Andrade, M.M.; Alberton, O.; Gonçalves, J.E.; Gazim, Z.C.; Magalhães, H.M. Substrate-associated mycorrhizal fungi promote changes in terpene composition, antioxidant activity, and enzymes in Curcuma longa L. acclimatized plants. Rhizosphere 2020, 13, 100191. [Google Scholar] [CrossRef]
- De Ferrari, M.P.S.; da Cruz, R.M.S.; dos Queiroz, M.S.; de Andrade, M.M.; Alberton, O.; Magalhães, H.M. Efficient ex vitro rooting, acclimatization, and cultivation of Curcuma longa L. from mycorrhizal fungi. J. Crops Sci. Biotechnol. 2020, 23, 469–482. [Google Scholar] [CrossRef]
- Blázquez, M.A. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Kundu, A. Environment friendly pesticides based on essential oils and their constituents. In Pesticides and Pests; Parmar, B.S., Singh, S.B., Walia, S., Eds.; Cambridge Scholars Publishin: Newcastle, UK, 2019; pp. 241–263. [Google Scholar]
- Garay, J. Review of essential oils: A viable pest control alternative. J. Hum. Ecol. 2020, 71, 13–22. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Pelagia Res. Libr. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Salas, M.L.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amza, J. Seed borne fungi; food spoilage, negative impact and their management: A review. Food Sci. Qual. Manag. 2018, 81, 70–79. [Google Scholar]
- Sawicka, B.; Egbuna, C. Pests of agricultural crops and control measures. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- WHO. Aflatoxins. Available online: https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf (accessed on 18 September 2020).
- Abbas, M.; Naz, S.A.; Shafique, M.; Jabeen, N.; Abbas, S. Fungal contamination in dried fruits and nuts: A possible source of mycosis and mycotoxicosis. Pak. J. Bot. 2019, 51, 1523–1529. [Google Scholar] [CrossRef]
- Tournas, V.H. Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit. Rev. Microbiol. 2005, 31, 33–44. [Google Scholar] [CrossRef]
- Kumar, V.; Iqbal, N. Post-harvest pathogens and disease management of horticultural crop: A brief review. Plant Arch. 2020, 20, 2054–2058. [Google Scholar]
- Schmidt, M.; Zannini, E.; Lynch, K.M.; Arendt, E.K. Novel approaches for chemical and microbiological shelf life extension of cereal crops. Crit. Rev. Food Sci. Nutr. 2019, 59, 3395–3419. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal agents in agriculture: Friends and foes of public health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Najafi, M.B.H.; Farhoosh, R. Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Viayaraghavan, P.; Ilavenil, S.; Al-Dhabi, N.A.; Choi, K.C. Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2019, 133, 54–62. [Google Scholar] [CrossRef]
- Atif, M.; Ilavenil, S.; Devanesan, S.; AlSalhi, M.S.; Choi, K.C.; Vijayaraghavan, P.; Alfuraydi, A.A.; Alanazi, N.F. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2020, 147, 112239. [Google Scholar] [CrossRef]
- Singh, G.; Singh, O.P.; Maurya, S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Prog. Cryst. Growth Charact. 2002, 45, 75–81. [Google Scholar] [CrossRef]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Panda, P.C.; Nayak, S. Deeper insight into the volatile profile of essential oil of two Curcuma species and their antioxidant and antimicrobial activities. Ind. Crops Prod. 2020, 155. [Google Scholar] [CrossRef]
- Péret-Almeida, L.; da Naghetini, C.C.; de Nunan, E.A.; Junqueira, R.G.; Glória, M.B.A. In vitro antimicrobial activity of the ground rhizome, curcuminoid pigments and essential oil of Curcuma longa L. Cienc. e Agrotecnol. 2008, 32, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Kavas, N.; Kavas, G. Use of turmeric (Curcuma longa L.) essential oil added to an egg white protein powder-based film in the storage of Çökelek cheese. J. Food Chem. Nanotechnol. 2017, 3, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Jeleń, H.; Wąsowicz, E. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 1998, 14, 391–426. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, J.; Kong, W.; Zhang, J.; Logrieco, A.F.; Wang, X.; Yang, M. Uncovering the antifungal components from turmeric (Curcuma longa L.) essential oil as Aspergillus flavus fumigants by partial least squares. RSC Adv. 2015, 5, 41967–41976. [Google Scholar] [CrossRef]
- Saju, K.A.; Venugopal, M.N.; Mathew, M.J. Antifungal and insect-repellent activities of essential oil of turmeric (Curcuma longa L.). Curr. Sci. 1998, 75, 660–662. [Google Scholar]
- Marin, S.; Guynot, M.E.; Sachnis, V.; Arbones, J.; Ramos, A.J. Aspergillus flavus, Aspergillus niger, and Penicillium corylophilum spoilage prevention of bakery products by means of weak-acid preservatives. Food Microbiol. Saf. 2002, 67, 2271–2277. [Google Scholar] [CrossRef]
- Gougouli, M.; Koutsoumanis, K.P. Risk assessment of fungal spoilage: A case study of Aspergillus niger on yogurt. Food Microbiol. 2017, 65, 264–273. [Google Scholar] [CrossRef]
- Mustapha, F.A.; Jai, J.; Raikhan, N.H.N.; Sharif, Z.I.M.; Yusof, N.M. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Velasco, J.A.C.; Mahecha, P.V.; Andrade-Mahecha, M.M. Essential oil turmeric (Curcuma longa) antifungal agent as in edible coatings applied to pumpkin minimally processed. Rev. Ciências Agrárias 2017, 40, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Oranusi, S.; Olarewaju, S. Mycoflora and aflatoxin contamination of some foodstuffs. Int. J. Biotechnol. Allied Fields 2013, 1, 9–18. [Google Scholar]
- Embaby, E.M.; Awni, N.M.; Abdel-galil, M.M.; El-gendy, H.I. Mycoflora and mycotoxin contaminated some juices. J. Agric. Technol. 2015, 11, 693–712. [Google Scholar]
- Saleem, A.R. Mycobiota and molecular detection of Aspergillus flavus and A. parasiticus aflatoxin contamination of strawberry (Fragaria ananassa Duch.) fruits. Arch. Phytopathol. Plant Prot. 2017, 50, 982–996. [Google Scholar] [CrossRef]
- García-Díaz, M.; Patiño, B.; Vázquez, C.; Gil-Serna, J. A novel niosome-encapsulated essential oil formulation to prevent Aspergillus flavus growth and aflatoxin contamination of maize grains during storage. Toxins 2019, 11, 646. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Tang, X.; Shen, Z.; Yang, K.; Zhao, L.; Li, Y. Comprehensive comparison of multiple quantitative near-infrared spectroscopy models for Aspergillus flavus contamination detection in peanut. J. Sci. Food Agric. 2019, 99, 5671–5679. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.F.; Gibbons, J.G.; Lee, M.K.; Han, K.H.; Hong, S.B.; Yu, J.H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.D.; Mossini, S.A.G.; Ferreira, F.M.D.; Arrotéia, C.C.; Da Costa, C.L.; Nakamura, C.V.; Junior, M.M. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci. World J. 2013, 2013, 34380. [Google Scholar] [CrossRef] [Green Version]
- Miedaner, T.; Gwiazdowska, D.; Waśkiewicz, A. Management of Fusarium Species and Their Mycotoxins in Cereal Food and Feed; Frontiers Media SA: Lausanne, Switzerland, 2017; p. 8543. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.F.; Mohd, M.H.; Nor, N.M.I.M.; Zakaria, L. Mycotoxigenic potential of Fusarium species associated with pineapple diseases. Arch. Phytopathol. Plant Prot. 2020, 53, 217–229. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology; Moretti, A., Sueca, A., Eds.; Springer: New York, NY, USA, 2017; Volume 1542, pp. 51–106. [Google Scholar] [CrossRef]
- Kumar, K.N.; Venkataramana, M.; Allen, J.A.; Chandranayaka, S.; Murali, H.S.; Batra, H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT Food Sci. Technol. 2016, 69, 522–528. [Google Scholar] [CrossRef]
- Senouci, H.; Benyelles, N.G.; Dib, M.E.A.; Costa, J.; Muselli, A. Chemical composition and combinatory antifungal activities of Ammoides verticillata, Allium sativum and Curcuma longa essential oils against four fungi responsible for tomato diseases. Comb. Chem. High Throughput Screen. 2020, 23, 196–204. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Yusof, N.M.; Jai, J.; Hamzah, F. Effect of coating materials on the properties of chitosan-starch-based edible coatings. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 507, p. 012011. [Google Scholar] [CrossRef]
- Bhadoria, P. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2010, 1, 7–20. [Google Scholar] [CrossRef]
- Chappell, M.; Knox, G.; Stamps, R.H. Alternatives to Synthetic Herbicides for Weed Management in Container Nurseries; The Institute of Food and Agricultural Sciences (IFAS): Quincy, FL, USA, 2012; pp. 1–6. [Google Scholar]
- Busi, R.; Vila-Aiub, M.M.; Beckie, H.J.; Gaines, T.A.; Goggin, D.E.; Kaundun, S.S.; Lacoste, M.; Neve, P.; Nissen, S.J.; Norsworthy, J.K.; et al. Herbicide-resistant weeds: From research and knowledge to future needs. Evol. Appl. 2013, 6, 1218–1221. [Google Scholar] [CrossRef]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of essential oils as bioherbicides. J. Essent. Oil-Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides—Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A.J., Kelto, J.A., Eds.; IntechOpen: London, UK, 2013; pp. 517–542. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Bessette, S.M. Herbicidal Composition Containing Plant Essential Oils and Mixtures or Blends Thereof. U.S. Patent 2002/0193250 A1, 19 December 2002. [Google Scholar]
- Symeonidou, A.; Petrotos, K.; Vasilakoglou, I.; Gkoutsidis, P.; Karkanta, F.; Lazaridou, A. Natural Herbicide Based on Essential Oils and Formulated as Wettable Powder. Patent EP 2684457 A1, 15 January 2014. [Google Scholar]
- CABI. Portulaca oleracea. Available online: https://www.cabi.org/isc/datasheet/43609 (accessed on 29 September 2020).
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [Green Version]
- DiTomaso, J.M.; Kyser, G.B. Italian and Perennial Ryegrass; Weed Research and Information Center: Davis, CA, USA, 2013; pp. 1–3. [Google Scholar]
- CABI. Lolium multiflorum (Italian Ryegrass). Available online: https://www.cabi.org/isc/datasheet/74001 (accessed on 29 September 2020).
- Bajwa, A.A.; Jabran, K.; Shahid, M.; Ali, H.H.; Chauhan, B.S. Ehsanullah Eco-biology and management of Echinochloa crus-galli. Crops Prot. 2015, 75, 151–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, T.; Zhao, B.; Yang, X.; Peng, Q.; Li, Y. Effects of common Echinochloa varieties on grain yield and grain quality of rice. Field Crops Res. 2017, 203, 163–172. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Ginger and turmeric essential oils for weed control and food crop protection. Plants 2019, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.; Singh, P.; Kedia, A.; Singh, A.; Dubey, N.K. Efficacy of essential oil combination of Curcuma longa L. and Zingiber officinale Rosc. As a postharvest fungitoxicant, aflatoxin inhibitor and antioxidant agent. J. Food Saf. 2012, 32, 279–288. [Google Scholar] [CrossRef]
- De Melo, S.C.; De Sá, L.E.C.; de Oliveira, H.L.M.; Trettel, J.R.; da Silva, P.S.; Gonçalves, J.E.; Gazim, Z.C.; Magalhães, H.M. Chemical constitution and allelopathic effects of Curcuma zedoaria essential oil on lettuce achenes and tomato seeds. Aust. J. Crop Sci. 2017, 11, 906–916. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Cortaderia selloana. Available online: http://www.iucnisd.org/gsid/species.php?sc=373 (accessed on 29 September 2020).
- Comité Científico; Comité de Flora y Fauna Silvestres. Solicitud de Dictamen Por Parte de la Junta de Extremadura Sobre la Pertinencia de la Denegación de la Propuesta de una Empresa que Pretende Llevar a Cabo un "Ensayo de Investigación con Nicotiana Glauca- TAPCS1 Como Cultivo Energético Para la Producción; Ministerio de Agricultura, Alimentación y Medio Ambient: Madrid, Spain, 2016; pp. 1–6.
- Estrategia de Gestión, Control y Posible Erradicación del Plumero de la Pampa (Cortaderia selloana) y otras Especies de Cortaderia. Available online: https://www.miteco.gob.es/es/biodiversidad/publicaciones/estrategia_cortaderia_tcm30-478424.pdf (accessed on 29 September 2020).
- Khattak, S.; Rehman, S.U.; Shah, H.U.; Ahmad, W.; Ahmad, M. Biological effects of indigenous medicinal plants Curcuma longa and Alpinia galanga. Fitoterapia 2005, 76, 254–257. [Google Scholar] [CrossRef]
- Abbasi, K.; Shah, A.A. Biological evaluation of turemeric (Curcuma longa). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 236–249. [Google Scholar]
- Akter, J.; Islam, M.Z.; Takara, K.; Hossain, M.A.; Gima, S.; Hou, D.X. Plant growth inhibitors in turmeric (Curcuma longa) and their effects on Bidens pilosa. Weed Biol. Manag. 2018, 18, 136–145. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 21–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Valadez-Blanco, R.; Hernández-Carlos, B.; Guadarrama-Mendoza, P.C. Natural antioxidant extracts as food preservatives. Acta Sci. Pol. Technol. Aliment. 2017, 16, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Tajiani, Z.; Jafari, S.M. Improving the shelf-life of food products by nano/micro-encapsulated ingredients. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier Inc.: London, UK, 2019; pp. 159–200. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Hosseini, H. Effects of turmeric, shallot extracts, and their combination on quality characteristics of vacuum-packaged rainbow trout stored at 4 ± 1 °C. J. Food Sci. 2011, 76, 387–391. [Google Scholar] [CrossRef]
- Gul, P.; Bakht, J. Antimicrobial activity of turmeric extract and its potential use in food industry. J. Food Sci. Technol. 2015, 52, 2272–2279. [Google Scholar] [CrossRef] [Green Version]
- Buch, S.; Pinto, S.; Aparnathi, K.D. Evaluation of efficacy of turmeric as a preservative in paneer. J. Food Sci. Technol. 2014, 51, 3226–3234. [Google Scholar] [CrossRef] [Green Version]
- Arulkumar, A.; Ramanchandran, K.; Paramasivam, S.; Palanivel, R.; Miranda, J.M. Effects of turmeric (Curcuma longa) on shelf life extension and biogenic amine control of cuttlefish (Sepia brevimana) during chilled storage. CYTA J. Food 2017, 15, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Al-Bahtiti, N.H. A study of preservative effects of turmeric (Curcuma longa) on mashed potatoes. Int. J. Appl. Eng. Res. 2018, 13, 4278–4281. [Google Scholar]
- Akbar, A.; Ali, I.; Samiullah; Ullah, N.; Khan, S.A.; Rehman, Z.; Rehman, S.U. Drought affects aquaporins gene expression in important pulse legume chickpea (Cicer arietinum L.). Pak. J. Bot. 2019, 51, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
- Pop, A.; Muste, S.; Paucean, A.; Chis, S.; Man, S.; Salanta, L.; Marc, R.; Muresan, A.; Martis, G. Herbs and spices in terms of food preservation and shelf life. Hop Med. Plants 2019, 27, 57–65. [Google Scholar]
- Nguyen, M.P. Synergistic effect of turmeric (Curcuma longa), galanga (Alpinia galanga) powder and lemongrass (Cymbopogon citratus) essential oil as natural preservative in chilled storage of white hard clam (Meretrix lyrata). Orient. J. Chem. 2020, 36, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.M.S.; Hoyos, C.G.; Velásquez-Cock, J.A.; Acosta, L.V.; Rojo, P.G.; Giraldo, A.M.V.; Gallego, R.Z. The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1842–1854. [Google Scholar] [CrossRef]
- Priya, R.; Prathapan, A.; Raghu, K.G.; Menon, A.N. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L. leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S695–S699. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [Green Version]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Li, Y. Reducing power: The measure of antioxidant activities of reductant compounds? Redox Rep. 2004, 9, 213–217. [Google Scholar] [CrossRef]
- Shahwar, D.; Raza, M.A.; Bukhari, S.; Bukhari, G. Ferric reducing antioxidant power of essential oils extracted from Eucalyptus and Curcuma species. Asian Pac. J. Trop. Biomed. 2012, 2, S1633–S1636. [Google Scholar] [CrossRef]
- Zaki, N.; Fa, M.; Yusof, N.; Jai, J. Turmeric (Curcuma longa L.) Oil as Antioxidant Agent in Starch-Based Edible Coating Film for Fresh-Cut Fruits. Available online: https://www.semanticscholar.org/paper/Turmeric-%28-Curcuma-longa-L-%29-Oil-as-Antioxidant-in-NAM-Mustapha/9404fbe5dcc4eef2313abf07d6a9b2d070f331d8?p2df (accessed on 19 November 2020).
- Ibáñez, M.D.; López-Gresa, M.P.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; González-Mas, M.C.; Blázquez, M.A. Essential oils as natural antimicrobial and antioxidant products in the agrifood industry. Nereis. Interdiscip. Ibero-Am. J. Methods Model. Simul. 2020, 12, 55–69. [Google Scholar] [CrossRef]
- Thakam, A.; Saewan, N. Chemical composition of essential oil and antioxidant activities of Curcuma petiolata Roxb. rhizomes. Adv. Mater. Res. 2012, 506, 393–396. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. https://doi.org/10.3390/plants10010044
Ibáñez MD, Blázquez MA. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants. 2021; 10(1):44. https://doi.org/10.3390/plants10010044
Chicago/Turabian StyleIbáñez, María Dolores, and María Amparo Blázquez. 2021. "Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review" Plants 10, no. 1: 44. https://doi.org/10.3390/plants10010044
APA StyleIbáñez, M. D., & Blázquez, M. A. (2021). Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants, 10(1), 44. https://doi.org/10.3390/plants10010044